Mechanistic Rationalization of Unusual Kinetics in Pd-Catalyzed C–H Olefination
Ryan D. Baxter, David Sale, Keary M. Engle, Jin-Quan Yu and Donna G. Blackmond
J. Am. Chem. Soc.,
2012, 4600-4606; 10.1021/ja207634t
02/2012
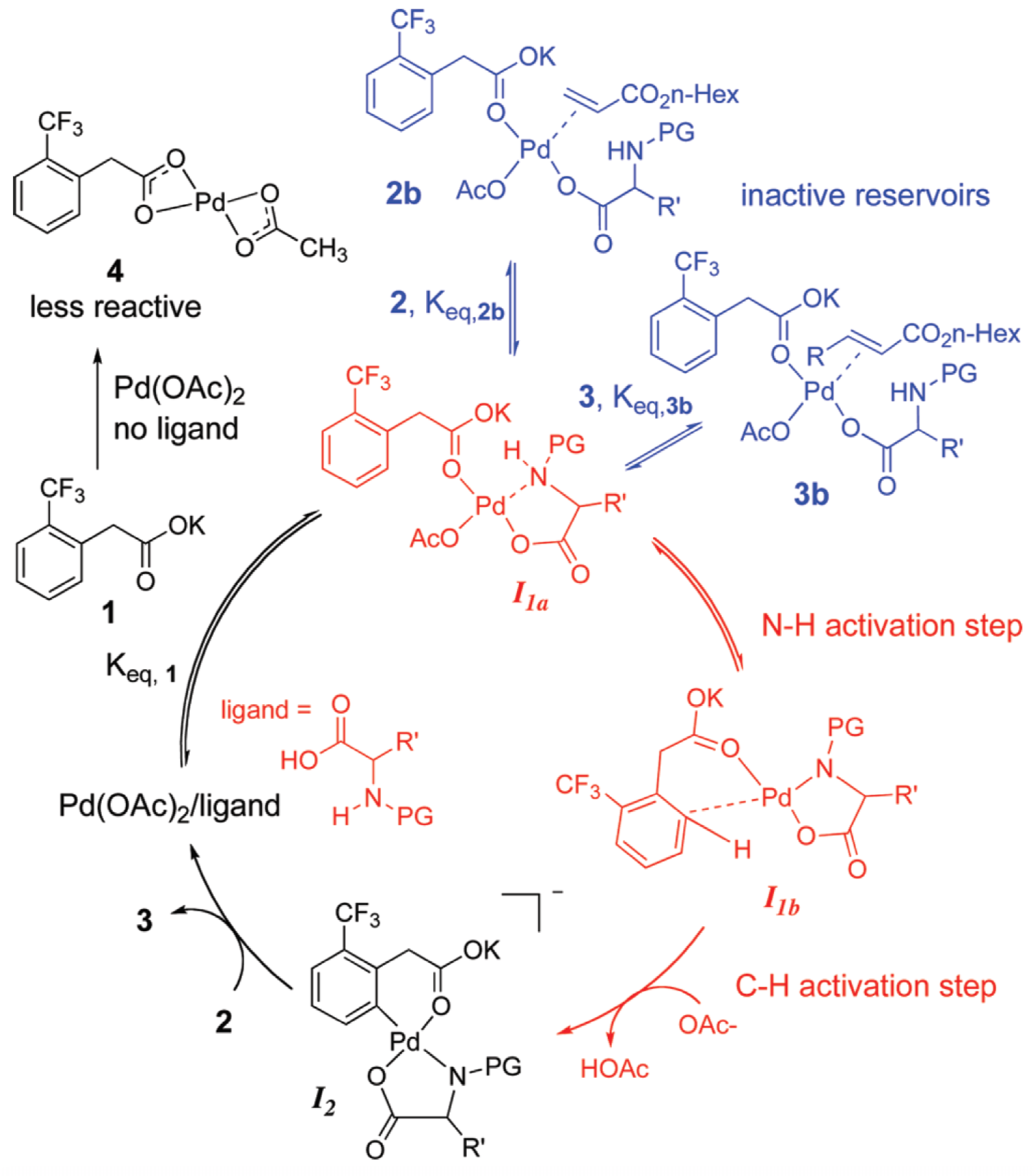
Detailed kinetic studies and novel graphical manipulations of reaction progress data in Pd(II)-catalyzed olefinations in the presence of mono-N-protected amino acid ligands reveal anomalous concentration dependences (zero order in o-CF3-phenylacetic acid concentration, zero order in oxygen pressure, and negative orders in both olefin and product concentrations), leaving the catalyst concentration as the sole positive driving force in the reaction.
NMR spectroscopic studies support the proposal that rate inhibition by the olefinic substrate and product is caused by formation of reversible off-cycle reservoirs that remove catalyst from the active cycle. NMR studies comparing the interaction between the catalyst and substrate in the presence and absence of the ligand suggest that weak coordination of the ligand to Pd prevents formation of an inactive mixed acetate species. A fuller understanding of these features may lead to the design of more efficient Pd(II) catalysts for this potentially powerful C–H functionalization reaction.