Understanding Reactivity and Stereoselectivity in Palladium-Catalyzed Diastereoselective sp3 C–H Bond Activation
Giri, R., Lan, Y., Liu, P., Houk, K. N., Yu, J.-Q.
J. Am. Chem. Soc.,
2012, 14118-14126; 10.1021/ja304643e
07/2012
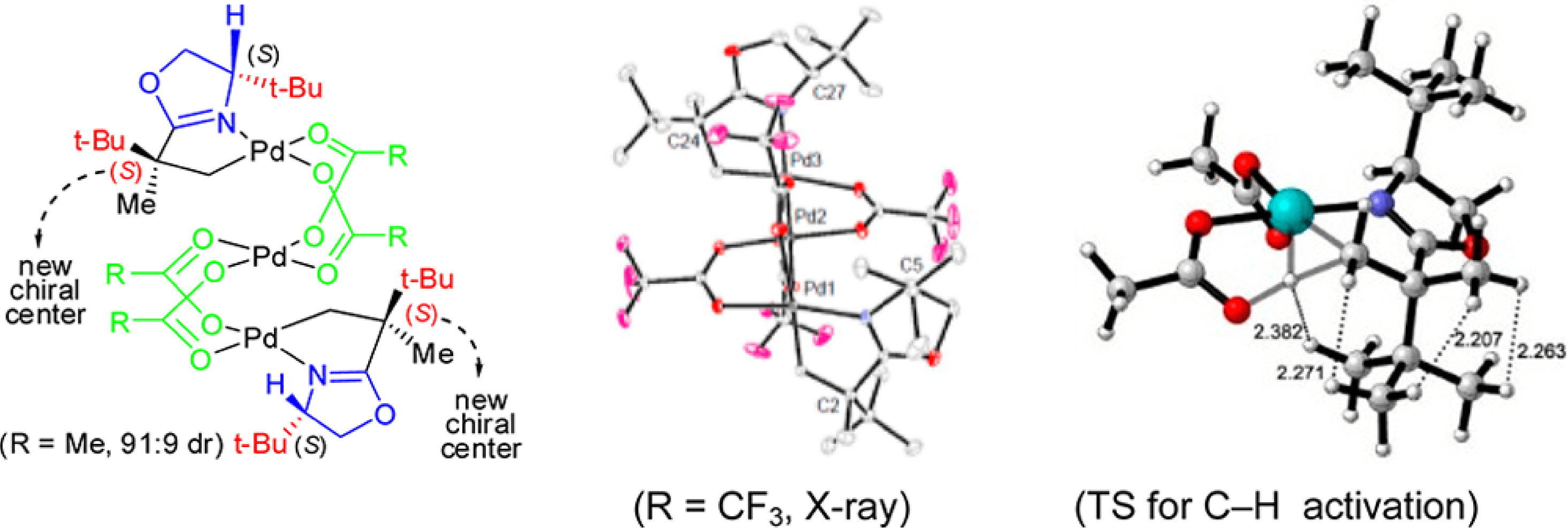
This collaborative project between the Yu and Houk groups has shed light on the origins of the high levels of reactivity and diastereoselectivity observed in the oxazoline directed Pd(II)-catalyzed sp3 C-H bond functionalization reactions.
Employing a combination of experimental and computational studies it was discovered that rather than the previously proposed trimeric species, it is the monomeric Pd species that is the active species. Theoretical predictions revealed that the most preferred transition state for the C-H activation contains two sterically bulky tert-Butyl substituents in the anti-position, due to steric repulsion, and that this transition state leads to the experimentally observed diastereomer.