C–H-Activated Direct Arylation of Strong Benzothiadiazole and Quinoxaline-Based Electron Acceptors
J. Zhang, T. C. Parker, W. Chen, L. Williams, V. N. Khrustalev, E. V. Jucov, S. Barlow, T. V. Timofeeva, and S. R. Marder
J. Org. Chem.,
2016, 81, (2), 360-370; 10.1021/acs.joc.5b02551
12/2015
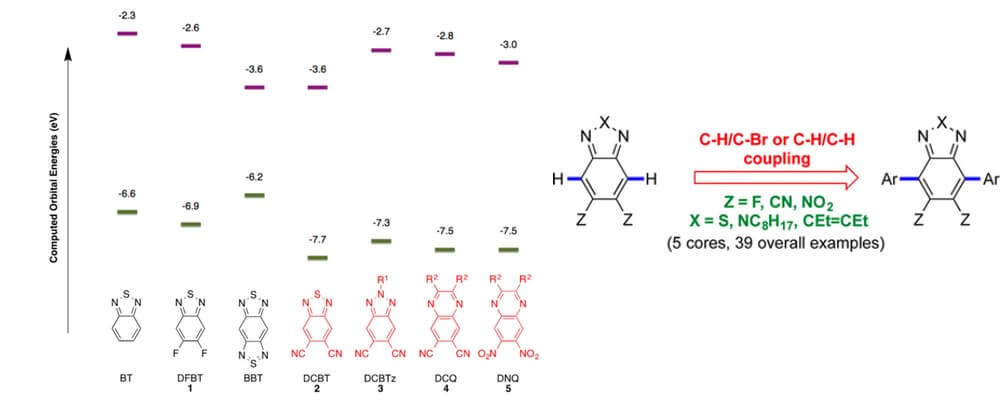
Organic pi-conjugated materials are foundational molecules for a variety of applications, including non-linear optics, organic photovoltaics and organic field-effect transistors. The fundamental physical properties of these systems can be tuned through structural variation.
Electron deficient molecular architectures are key components of conducting systems as they act as electron acceptors. However, incorporation of strong pi-acceptors into new materials can be problematic using more established Stille or Suzuki cross coupling methodologies. Many electron-poor precursors are resistant to electrophilic halogenation or require harsh and forcing conditions which can lead to low yields.
The Marder group, in collaboration with the Timofeeva group, identified C–H functionalization as a technology that offers the potential to address this shortcoming. Building upon a series of mechanistic studies performed by the Fagnou group that identified a higher reactivity for C–H bonds on electron-accepting substrate, a direct arylation strategy that provides rapid access to a broad range of strong benzothiadiazole and quinoxaline-based electron acceptors for incorporation into materials.
This report discloses the optimization and exploration of this transformation and the investigation of the properties of these new material building blocks.