Hapalindole/Ambiguine Biogenesis Is Mediated by a Cope Rearrangement, C–C Bond-Forming Cascade
S. Li, A. N. Lowell, F. Yu, A. Raveh, S. A. Newmister, N. Bair, J. M. Schaub, R. M. Williams, and D. H. Sherman
J. Am. Chem. Soc.,
2015, 137, (49), 15366-15369; 10.1021/jacs.5b10136
12/2015
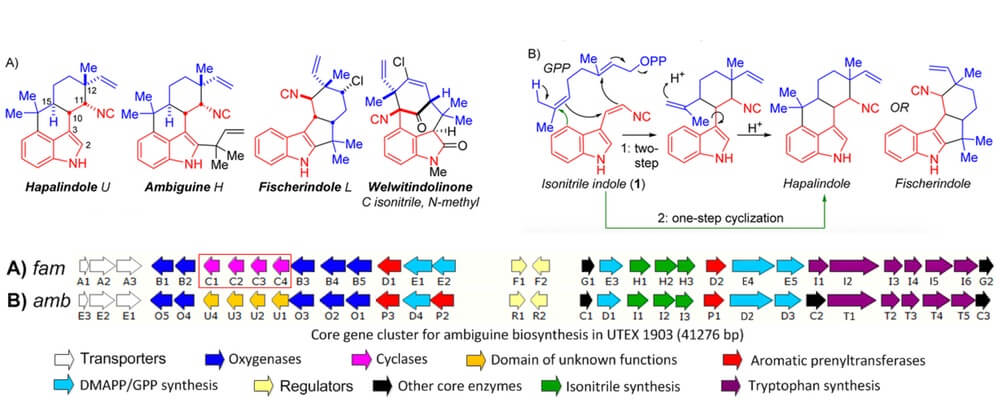
The Hapalindoles are a large class of structurally fascinating indole alkaloid natural products that have expressed significant biological activity, making them targets of exceptional interest within the chemical community. While there have been a number of successful synthetic campaigns toward these compounds there has been limited research concerning the biogenesis of these terpenoids.
This collaborative study from the Sherman and Williams groups employs an array of tools to gather evidence for a novel mechanistic proposal.
Through a detailed examination of the gene cluster from Fischerella ambigua UTEX 1903, the Sherman group were able to identify a number of the key enzymes involved in the biosynthetic pathway, including a number of cyclases that support the novel proposal of a Cope-rearrangement, C-C bond forming cascade to stitch together carbon framework of these molecules.
This proposal is particularly significant as this represents one of the only biosynthetic Cope rearrangements. While there has been much discussion around this hypothesis these findings lay the groundwork for many further studies in this area.