Oxygen Activation by Co(II) and a Redox Non-Innocent Ligand: Spectroscopic Characterization of a Radical–Co(II)–Superoxide Complex with Divergent Catalytic Reactivity
A. R. Corcos, O. Villanueva, R. C. Walroth, S. K. Sharma, J. Bacsa, K. M. Lancaster, C. E. MacBeth, and J. F. Berry
J. Am. Chem. Soc.,
2016, 138 (6), pp 1796–1799; 10.1021/jacs.5b12643
01/2016
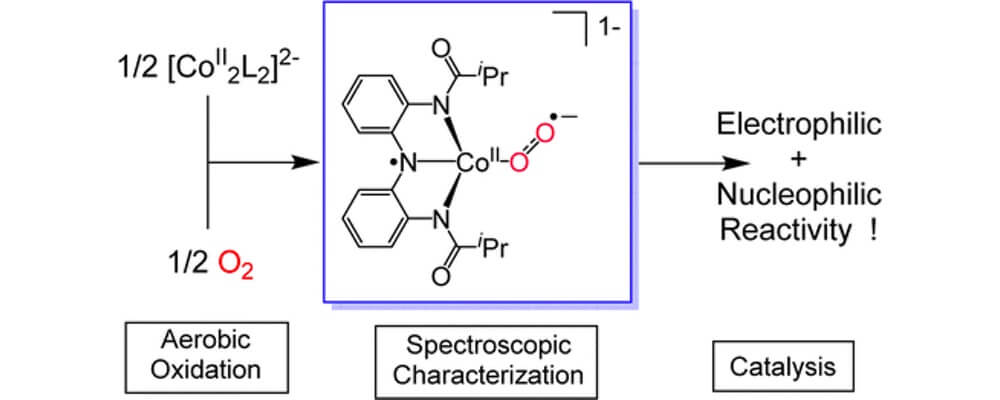
Synthetic cobalt complexes have been known to bind to molecular oxygen for over 100 years. However, upon addition of oxygen most Co(II) complexes form kinetically inert low-spin Co(III) terminal superoxides or peroxide compounds that are catalytically inactive. Active compounds can be synthetically generated through the introduction of a coreductant, but in nature redox non-innocent ligands are employed to supply the electrons when activating oxygen.
This collaborative report from the Berry, MacBeth and Lancaster groups take inspiration from nature and explore a recently reported dimeric Co(II) complex that has shown some interesting reactivities.
Using a panel of techniques, including EPR, K-edge XANES and DFT-simulations, the properties of this Cobalt system are explored and the origins of the unusual divergent electrophilic and nucleophilic reactivity of this system are deduced.
Understanding the role of the non-innocent redox ligands in the stabilization of these complexes is one of the key factors for the development of catalysts based on first row transition metals.