Cu-Catalyzed aromatic C–H imidation with N-fluorobenzenesulfonimide: mechanistic details and predictive models
Brandon E. Haines, Takahiro Kawakami, Keiko Kuwata, Kei Murakami, Kenichiro Itami and Djamaladdin G. Musaev
Chemical Science,
2017, 8, 988-1001; DOI: 10.1039/C6SC04145K
10/2016
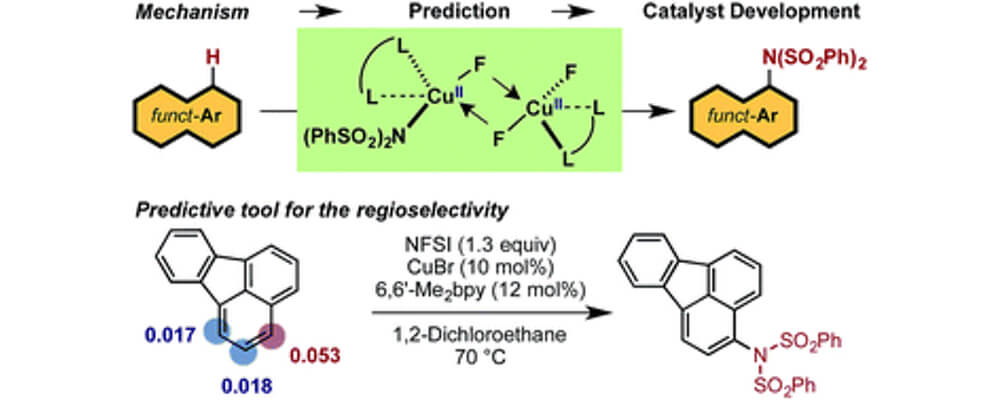
This collaborative report from the Musaev (Emory University) and Itami (ITbM, Nagoya University, Japan) groups describes the mechanistic investigation of this transformation and how the understanding developed from these studies has been used to develop a predictive model that was validated and refined in these studies.
This collaboration came about as a result of the CCHF’s Science Across Virtual Institutes (SAVI) program, that brings together collaborators from across the globe to address issues in C–H Functionalization.
The copper-catalyzed C–H imidation of arenes using N-fluorobenzenesulfonimide (NFSI), was previously reported by the Itami group was notable as it employed abundant metals for the catalysis and was effective across a broad scope of complex arenes.
The theoretical studies disclosed in this report detail the two main stages of the reaction; the generation of the active catalyst and the catalytic cycle. With a detailed understanding of these two steps it was possible to develop a predictive tool that was experimentally validated capable of determining the regiochemical outcome for a given substrate.
One of the goals of the CCHF is to bring C–H functionalization into the mainstream of organic chemistry. This report speaks to this in two ways, the development of an effective and applicable transformation, along with a predictive and reliable tool that can be used by those not yet expert in C–H functionalization.