Mechanism, Regio-, and Diastereoselectivity of Rh(III)-Catalyzed Cyclization Reactions of N-Arylnitrones with Alkynes: A Density Functional Theory Study
Yingzi Li, Chunhui Shan, Yun-Fang Yang, Fuqiang Shi, Xiaotian Qi, K. N. Houk, and Yu Lan
J. Phys. Chem. A.,
2017, 121, (23), 4496-4504; DOI: 10.1021/acs.jpca.7b01020
05/2017
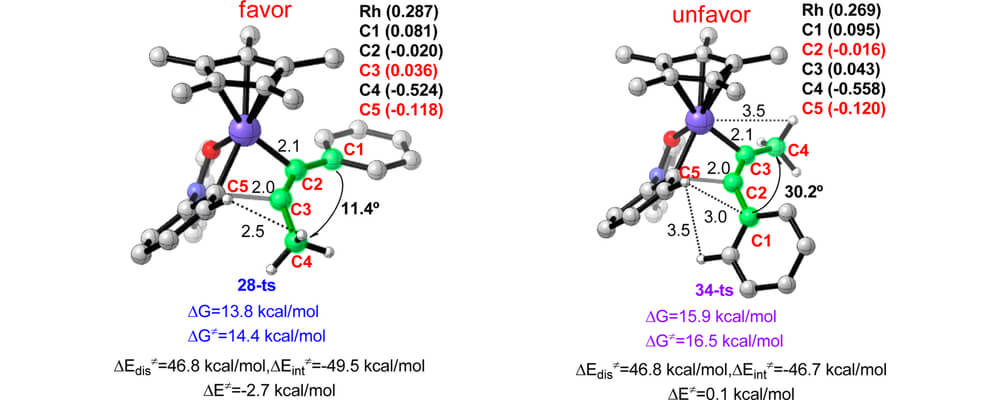
Nitrones have been used as effective substrates in the rhodium-catalyzed cyclization/C–H bond activation and O atom transfer of arylnitrones with alkynes. In this collaborative study between the Houk and Lan groups, the mechanism of this transformation is studied.
Results from the DFT calculations outline the reaction pathway for this system, and highlight that the regioselectivity of this reaction with asymmetric alkynes is controlled by the electronic nature of the substituents.