Entrances, Traps, and Rate-Controlling Factors for Nickel-Catalyzed C−H Functionalization
Alex J. Nett , John Montgomery, and Paul M. Zimmerman
ACS Catalysis,
2017, 7, (10), 7352-7362; DOI:10.1021/acscatal.7b02919
09/2017
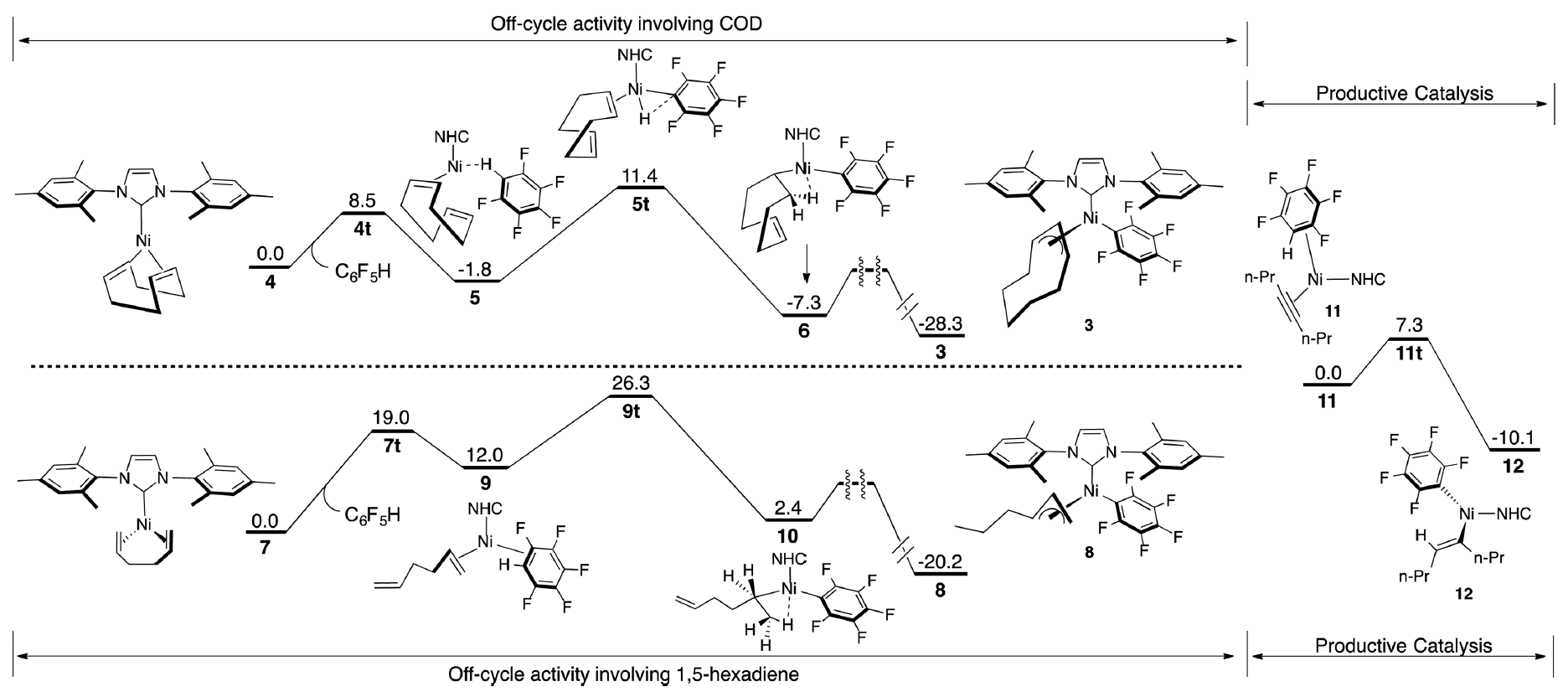
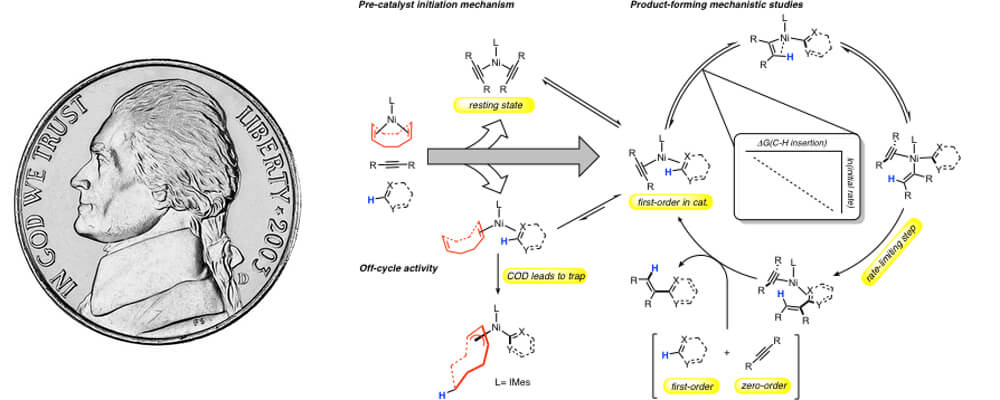
This publication is a collaborative research effort between Professor John Montgomery's group at the University of Michigan in the CCHF and external collaborator Professor Paul Zimmerman, also at the University of Michigan. The details of this report focus on a long-standing interest of these two groups in addressing the efficiency of nickel-catalyzed C-H functionalization processes. Developing a complete understanding of a catalytic process, including the productive catalytic cycle as well as catalyst activation steps and off-cycle competing pathways, presents a daunting challenge in mechanistic analysis. Through a combination of experimental and computational studies, a comprehensive description of the nickel-catalyzed hydroarylation of alkynes has been provided.
In this study, we observed that undesired off-cycle intermediates can form during the generation of active catalytic species and inhibit productive catalysis.. Analysis of the precatalyst activation steps led to the identification of a new precatalyst that diminished undesired activity, producing a highly active catalyst for C-H functionalization at room temperature. By examining the correlation of various kinetic and thermodynamic parameters with substrate scope and efficiency, the thermodynamics of metal-carbon bond strength of the species that follows C-H cleavage was found to be an excellent predictor of reaction efficiency. Using state-of-the-art computational methods, a complete profile of the initiation and on-cycle and off-cycle steps was described.