Isocanthine Synthesis via Rh(III)-Catalyzed Intramolecular C–H Functionalization
Anthony Y. Chen, Qianqian Lu, Yao Fu, Richmond Sarpong, Brian M. Stoltz, and Haiming Zhang
J. Org. Chem.,
2018, 83, (1), 330; DOI:10.1021/acs.joc.7b02731
01/2018
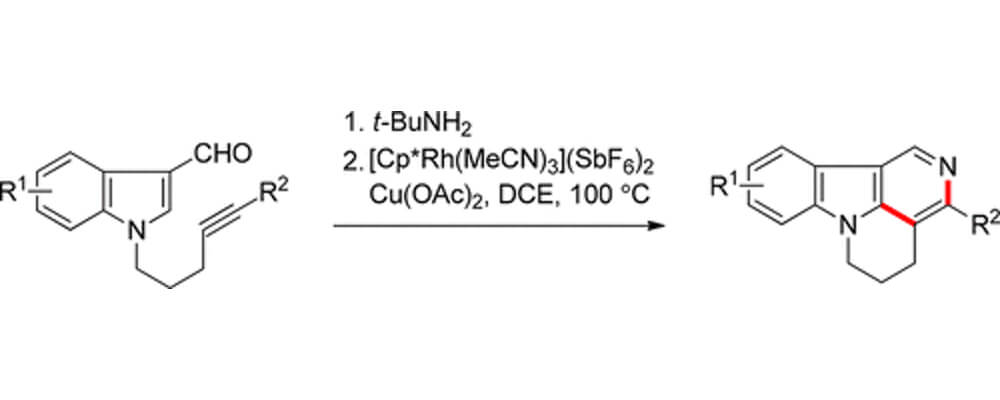
The Stoltz and Sarpong labs, in collaboration with Genentech and the University of Science and Technology of China, have developed a Rh(III)-catalyzed C–H functionalization method for synthesizing isocanthines from N-tethered indole-3-carboxaldehydes. Isocanthines are a class of annulated γ-carbolines that have been extensively studied for their clinical use as cardiovascular agents, as well as for their antiemetic properties.
The reported C–H functionalization method proceeds under mild reaction conditions and tolerates a variety of 5- or 6-substituted indole starting materials. The method can also be extended to synthesize annulated 5-azaindoles from pyrrole starting materials. Computational studies suggest the mechanism of this transformation involves ortho-directed C–H activation, followed by alkyne coordination and migratory insertion, then reductive elimination and tert-butyl fragmentation to afford the desired isocanthine product. Anthony Chen was an undergraduate summer intern in the Process Chemistry Department at Genentech where this collaborative project was initiated.
Author: Anthony Chen