Site-Selective Carbene-Induced C–H Functionalization Catalyzed by Dirhodium Tetrakis(triarylcyclopropanecarboxylate) Complexes
Kuangbiao Liao, Wenbin Liu, Zachary L. Niemeyer, Zhi Ren, John Bacsa, Djamaladdin G. Musaev, Mathew S. Sigman, and Huw M. L. Davies
ACS Catalysis,
2018, 8, (1), 678; DOI:10.1021/acscatal.7b03421
01/2018
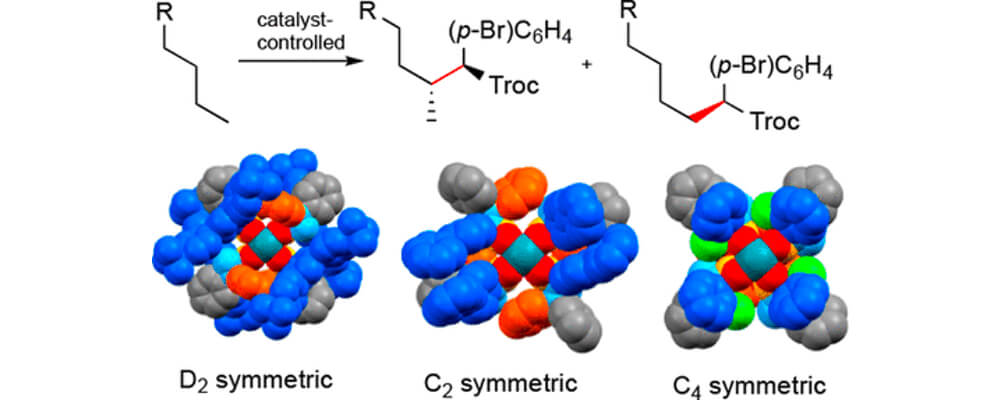
In this collaborative study between the Davies, Musaev and Sigman groups, we describe three dirhodium tetrakis(triarylcyclopropanecarboxylate) complexes [Rh2(TPCP)4 catalysts].
The catalysts can adopt three different high-symmetry orientations, which is dependent on the functionality on the cyclopropanes, as shown in the graph above. All three catalysts are effective in the C–H functionalization of unactivated C–H bonds by means of carbene-induced C–H insertion.
The reaction can occur even in the presence of functional groups. The D2 and C4 symmetric catalysts cause preferred reactions at the most sterically accessible secondary C–H bond, whereas the C2 symmetric catalyst enhances reaction at the most accessible primary C–H bond. Even though the three catalysts have different ligand arrangements, it is still possible to develop a quantitative model for the site selectivity exhibited by these catalysts.
Author: Wenbin Liu