Generality and Strength of Transition Metal β-Effects
Brandon E. Haines, Richmond Sarpong and Djamaladdin G. Musaev
J. Am. Chem. Soc.,
2018, 140 (33), pp 10612–10618; DOI:10.1021/jacs.8b06817
07/2018
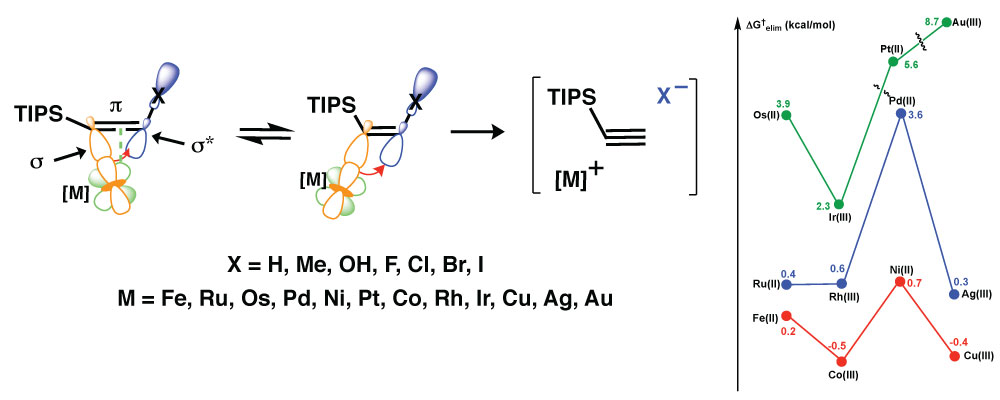
We provide a comprehensive theoretical and historical context for the beta-effects of transition metal ions, in particular Pd(II). It is shown that Pd(II) imparts a stronger beta-effect than Si to stabilize a cation through hyperconjugation (sigma-pi conjugation,) or in stabilizing the transition state for a departing nucleofuge (sigma-sigma conjugation). Due to the established connection between these modes of hyperconjugation, we analyzed the strength and generality of transition metal beta-effects in an experimentally relevant system, and estab-lished convenient and practical computational parameters to investigate the hyperconjugation for various X substituents on Cbeta–center and transition metals: (a) the degree elongation of Cbeta–X bond, relative to a reference with baseline levels of hyperconjugation; (b) the second-order perturbation energy, E(2)ij, extracted from the NBO analysis, (c) the energy gap between these orbitals, ΔE; and (d) the free ener-gy barrier for beta-elimination of the X group, ΔG‡elim. It is shown that the nature of a substituent (X) on Cbeta critically affects the sigma-sigma conjugation and, consequently, the energy barrier to vinyl beta-elimination. We find that the beta-Pd effect is relatively small for X = H, Me, OH and F, but significant for X = Cl, Br and I. We also shown that the first-row transition metals exhibit the strongest beta-effects.