Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation
Poonam Srivastava, Hao Yang, Ken Ellis-Guardiola and Jared C. Lewis
Nature Commun.
2015, 6, 7789; 10.1038/ncomms8789
07/2015
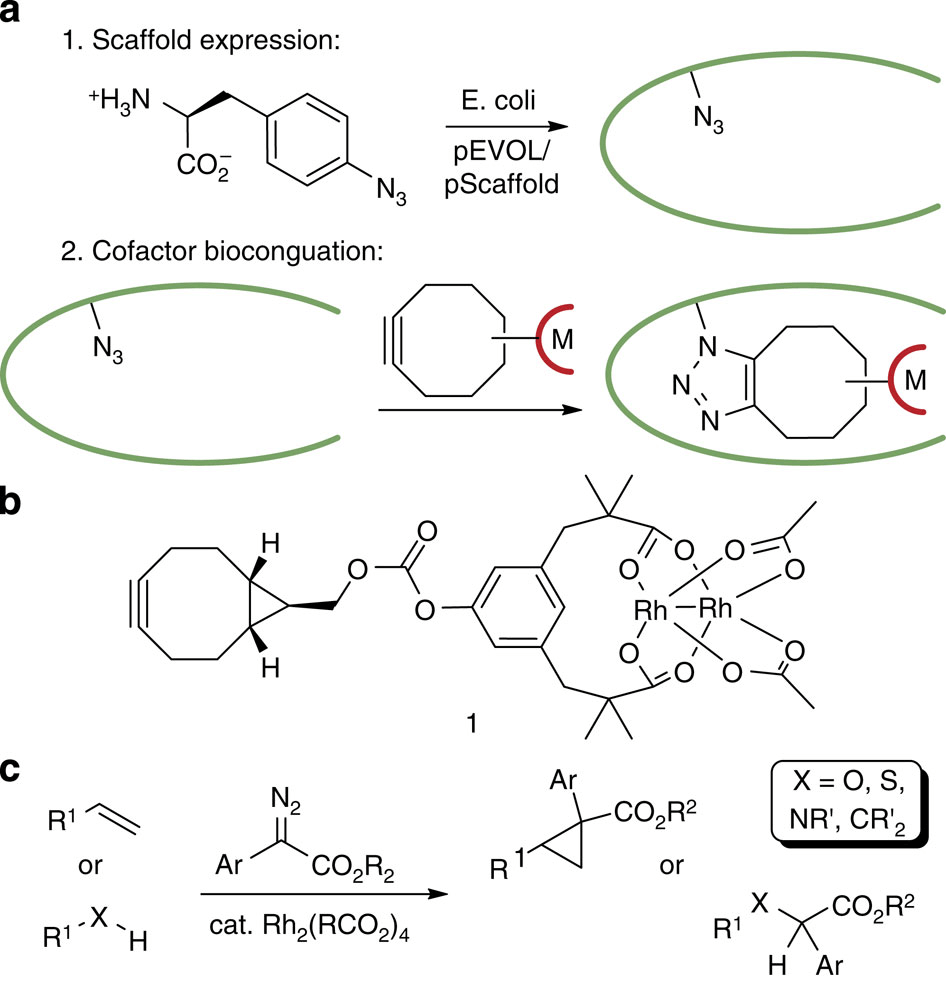
One of the key drives central to the research being done by the CCHF is the exploration of new approaches to modulate the selectivity and specificity of catalysts. As part of an ongoing program in the Center, this report describes the exploitation of the substrate-binding and activation capabilities of enzymes for reactions not found in Nature through a recently developed method to link synthetic catalysts and protein scaffolds to create artificial metalloenzymes (ArM’s).
The Lewis group have recently developed a new approach for ArM synthesis, taking advantage of a strain-promoted azide-alkyne cycloaddition (SPAAC) to couple the scaffold and cofactor, the key feature of which is that unlike most covalent methods the bioorthogonality of SPAAC eliminates the need to remove more reactive residues, significantly expanding the range of possible protein scaffold available.
This allows the use of prolyl oligiopeptidase (POP) scaffolds, attractive due to their shape and reactive volume. Optimization of these systems through genetic optimization of the protein scaffold and use of a dirhodium cofactor based on the Rh2(esp)2 developed by Du Bois, has furnished an ArM that is highly effective at asymmetric cyclopropanation, significantly suppresses side reaction with water and is effective across a broad range of substrates.
With the broad spectrum of reactions catalyzed by dirhodium complexes this novel dirhodium ArM manifold is anticipated to provide many opportunities for selective catalysis in the near future and with POP serving as a stable, robust and customizable scaffold this systems will provide an excellent opportunity to study the effects of attractive interactions, molecular recognition and scaffold dynamics on metal catalysis.