Syntheses of Isoquinoline and Substituted Quinolines in Charged Microdroplets
Shibdas Banerjee, Richard N. Zare
Angew. Chem. Int. Ed.,
2015, 54, 49, 14795-14799; 10.1002/anie.201507805
10/2015
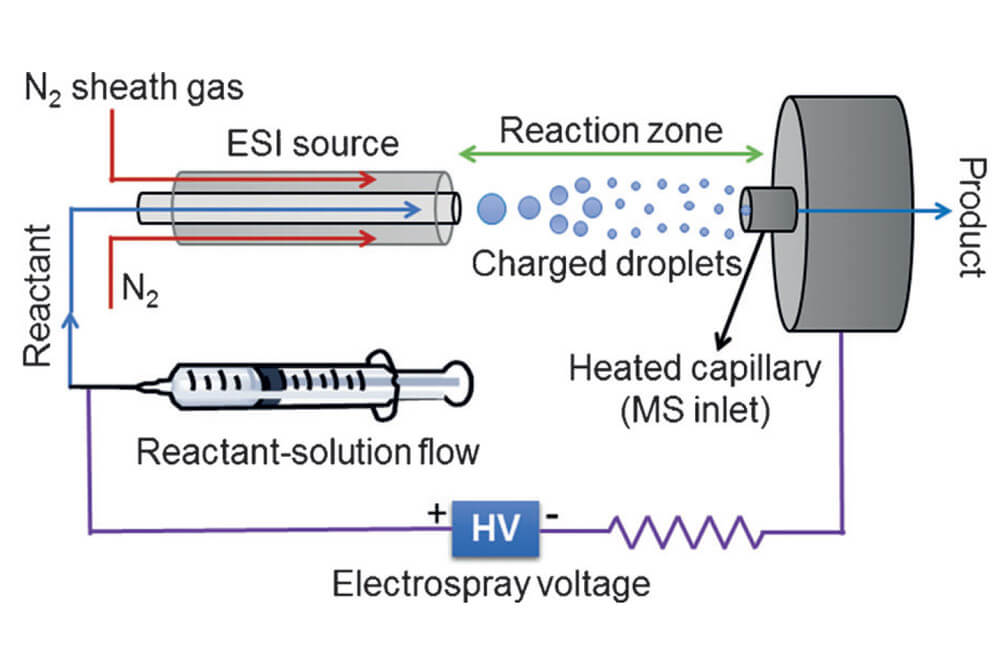
During the course of their studies in the development of ‘microdroplet fusion mass spectrometry’ in order to observe early and transient events in chemical and biochemical processes the Zare group have observed that rate of reaction performed in these microdroplets is significantly greater than that observed in more traditional bulk solutions.
This report deals with a particular aspect of this work, performing the synthesis of isoquinolines and substituted quinolones under these microdroplet conditions. Significantly the three reactions performed, the Pomeranz-Fritsch, Friedländer and Combes reactions typically take forcing acidic conditions (upto 70% concentrated sulphuric acid at over 100 oC) and between hours and days to complete. Performing these reactions in the charged microdroplets the transformation is complete in a matter of microseconds and at room temperature with no addition of acid.
The Zare group explore the effects and impacts of performing reaction under these new conditions and discuss possible rationales for how this reaction can be performed in the absence of acid under these new reaction conditions. This new technology is just in its infancy and investigations are underway to explore how this can be applied in new technologies such as C–H Functionalization.