Mono-Oxidation of Bidentate Bis-phosphines in Catalyst Activation: Kinetic and Mechanistic Studies of a Pd/Xantphos-Catalyzed C–H Functionalization
Yining Ji, R. Erik Plata, Christopher S. Regens, Michael Hay, Michael Schmidt, Thomas Razler, Yuping Qiu, Peng Geng, Yi Hsiao, Thorsten Rosner, Martin D. Eastgate, and Donna G. Blackmond
J. Am. Chem. Soc.,
2015, 137, (41), 13272-13281; 10.1021/jacs.5b01913
09/2015
Bidentate bis-phosphine ligands have become a privileged motif across a number of metal-mediated transformations including borylation, Suzuki cross-coupling, direct arylation, C–N amination and C–H functionalization. As a result of this widespread application there have been significant studies around the role these ligands play. However, little is known about how the metal/ligand complex enters into the catalytic cycle.
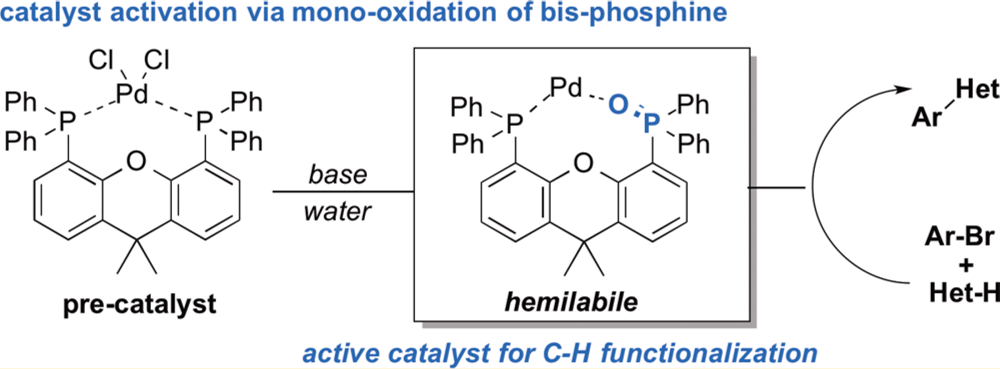
In this collaborative paper between the Blackmond Group and the Chemical Development group from Bristol-Myers Squibb a suite of techniques was brought to bear on this question. Employing kinetic, spectroscopic, crystallographic and computational studies are used to probe a Pd-catalyzed C–H arylation reaction. In previous studies it had been demonstrated that the reduction of the high-oxidation state pre-catalyst had significant implications for the structure and ability of the catalytically competent complexes. Even changing the order of addition of reagents fundamentally influenced both the catalyst structure and mechanism of the reaction.
Using this this combination of tools a highly detailed and rigorous picture of the catalytic cycle was developed, revealing that mono-oxidation of the bis-phosphine ligand is critical in the formation of the active catalyst and describing the import of this hemi-labile ligand system.
These studies have broad implications across the transformations for which these conditions and complex formation are common.