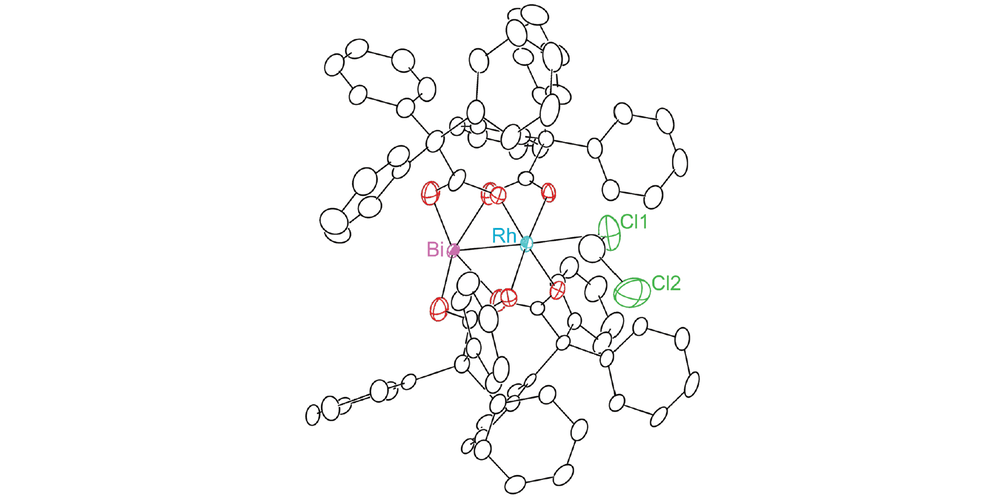
The first bismuth(II)–rhodium(II) oxypyridinate paddlewheel complexes: Synthesis and structural characterization
Travis Lee Sunderland and John F. Berry
J. Coord. Chem.,
2016, 69, 11-13, 1949-1957; 10.1080/00958972.2016.1177178
05/2016
Paddlewheel complexes are privileged scaffolds in C–H Functionalization chemistry, with the dirhodium and diruthenium systems providing some of the most effective catalysts for the formation of C–C and C–N bonds.
Recently the techniques for the the modular synthesis of heterometallic analogues have seen some major advances. This report from the Berry group describes the first synthesis and structural characterization of a heterobimetallic Rh-Bi complex supported by non-carboxylate ligands.
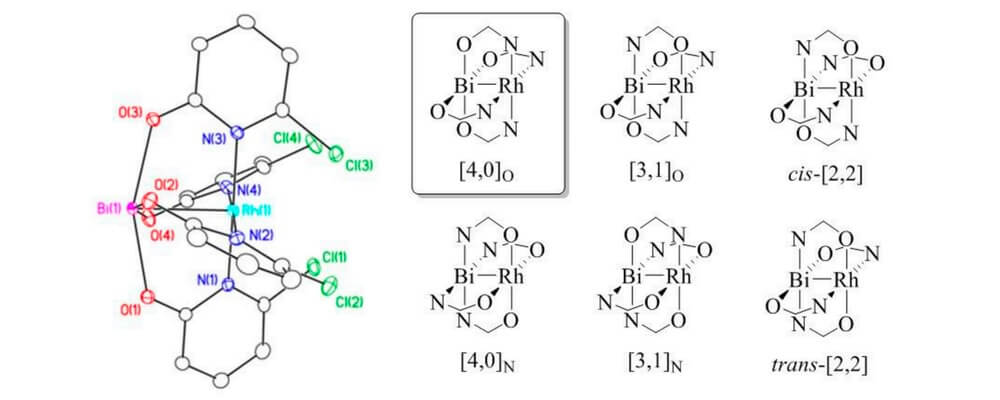
Using oxypyridinates to support the heterobimetallic Rh-Bi core six geometric isomers are possible. Interestingly the greater oxophilicity of the bismuth atom leads to the formation of a single geometric isomer.
This geometric preference has some significant implications for future work in this area. The specific steric and stereochemical orientation of the ligands that surround these paddlewheel complexes determine the site- and stereo-selectivity of the reactions they catalyze. Being able to generate a single geometric isomer, different from the homobimetallic Rh–Rh analogues presents a very interesting potential avenue of reaction investigation and development.