Site Selective and Stereoselective Functionalization of Unactivated C–H Bonds
Kuangbiao Liao, Solymar Negretti, Djamaladdin G. Musaev, John Bacsa and Huw M. L. Davies
Nature,
2016, 533, 230-234; 10.1038/nature17651
05/2016
The traditional strategy for the synthesis of complex organic compounds has relied on the use of functional groups, while the C–H bonds behave as unreactive bystanders. The idea of C–H functionalization, in which C–H bonds are modified at will instead of the functional groups, represents a paradigm shift in the standard logic of organic synthesis.
This collaborative report from the Davies and Musaev groups describes the use of dirhodium catalysts to achieve the first examples of highly site selective, diastereoselective and enantioselective C–H functionalization of n-alkanes and terminally-substituted n-alkyl compounds. Using a combined experimental and theoretical approach an understanding of the factors involved in the catalyst environment that impact site reaction is reached.
Related Content
-
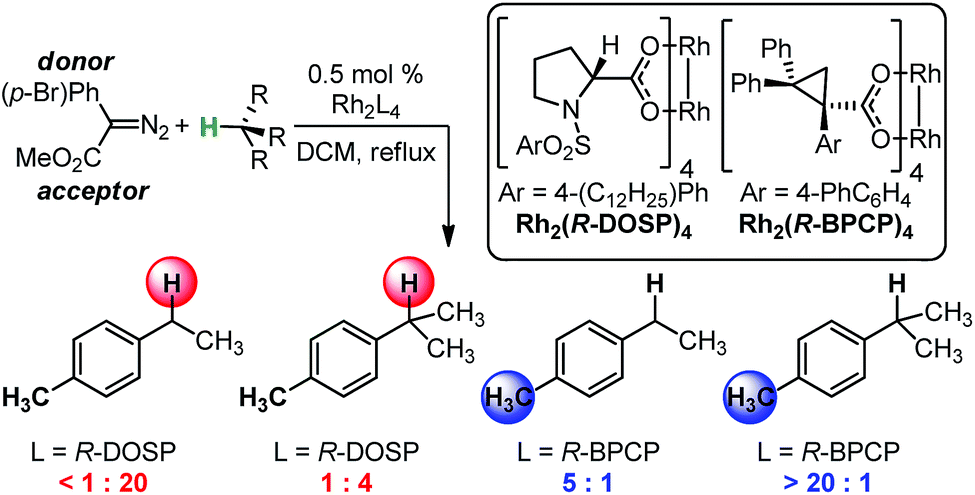
03/2015
Using IR vibrations to quantitatively describe and predict site-selectivity in multivariate Rh-catalyzed C–H functionalization
RESEARCH
-
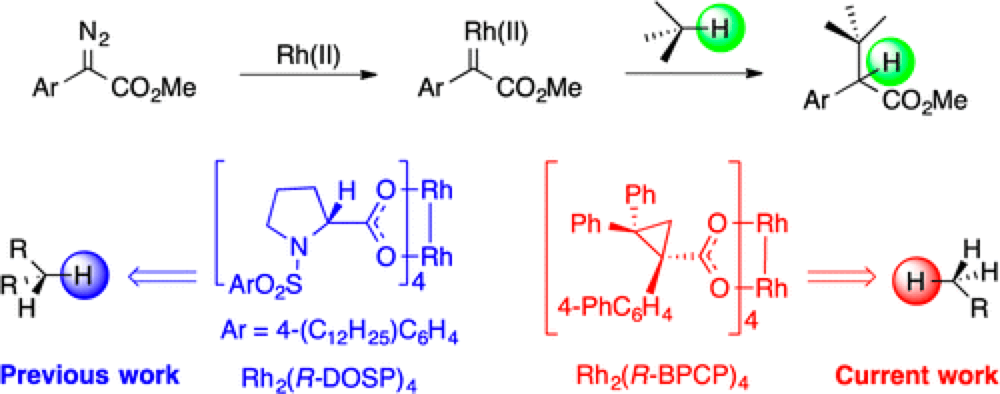
06/2014
Role of Sterically Demanding Chiral Dirhodium Catalysts in Site-Selective C–H Functionalization of Activated Primary C–H Bonds
RESEARCH
-
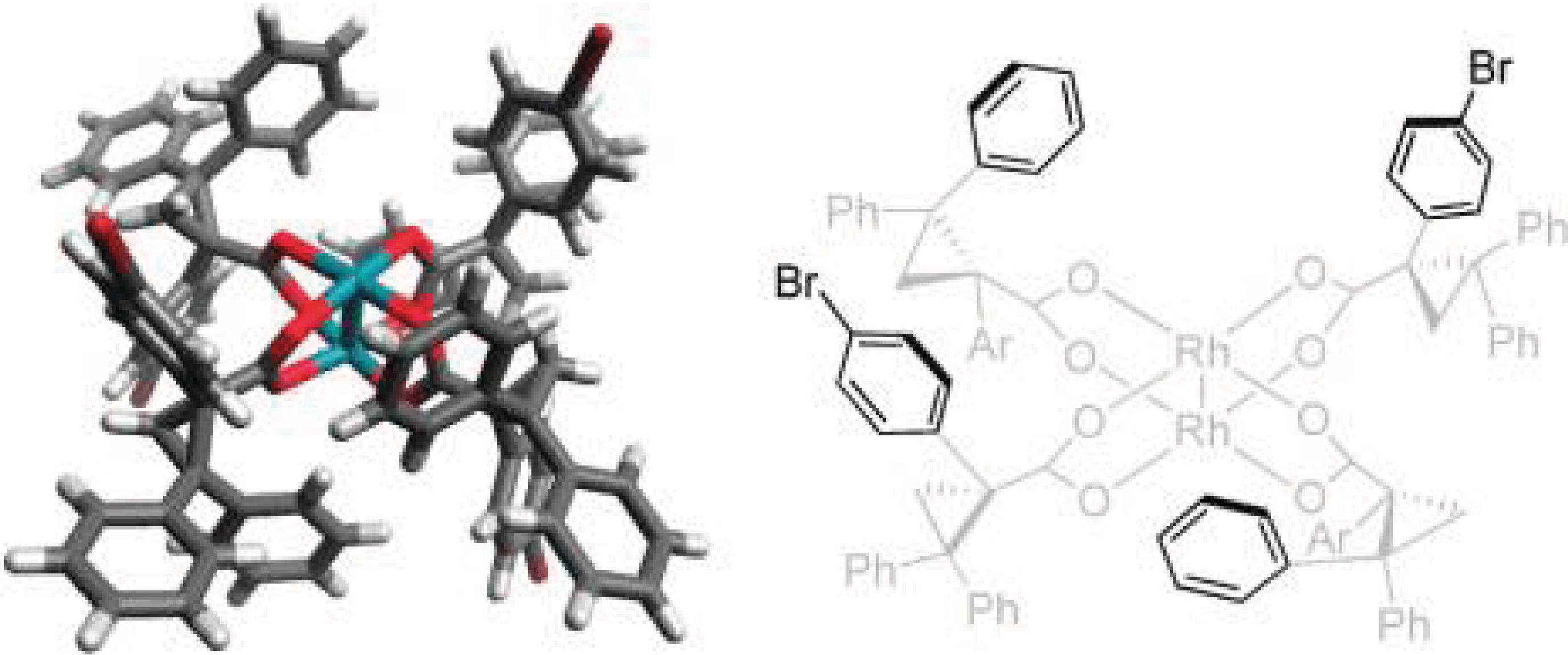
11/2011
D2-Symmetric Dirhodium Catalyst Derived from a 1,2,2-triarylcyclopropane carboxylate Ligand: Design, Synthesis and Application
RESEARCH