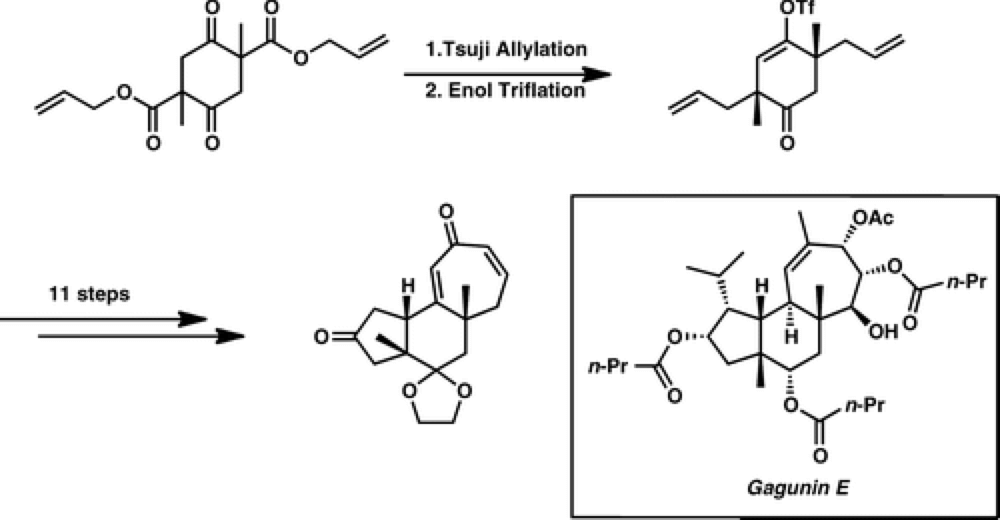
Enantioselective Synthesis of the 5–6–7 Carbocyclic Core of the Gagunin Diterpenoids
Grant M. Shibuya, John A. Enquist, Jr., Brian M. Stoltz
Organic Letters,
2013, 15, (13), 3480-3483; 10.1021/ol401514s
06/2013
The Stoltz group report an expedient entry into the carbocyclic core of gagunin class of diterpenoids, generating this complex polycyclic core in just seven steps.
Using a catalytic enantioselective double allylic alkylation to define the stereochemistry of the molecule. With this skeleton built substitution of the scaffold in a selective fashion would provide rapid access to many members of the natural product family. It also provides an exciting opportunity to test the C–H functionalization technology developed by the Center in a complex molecular scaffold.