The Uranyl Cation as a Visible-Light Photocatalyst for C(sp3)−H Fluorination
Julian G. West, T. Aaron Bedell, Erik J. Sorensen
Angew. Chem. Int. Ed.,
2016, 55, 31, 8923, DOI: 10.1002/anie.201603149
06/2016
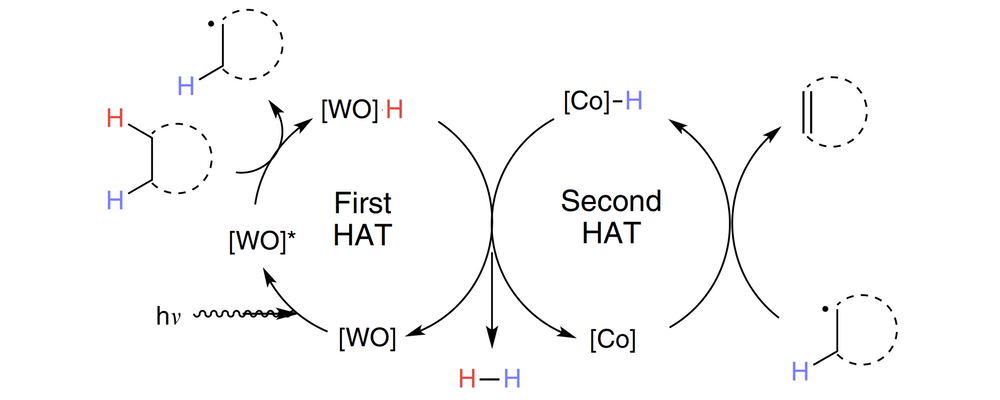
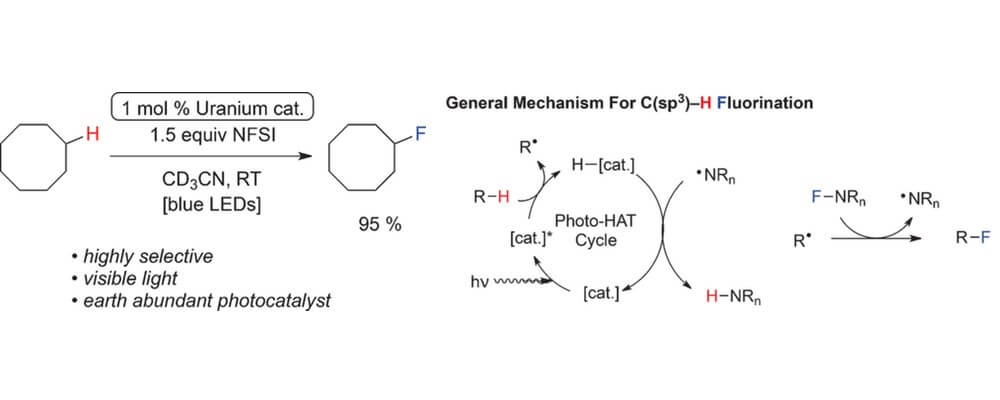
The fluorination of unactivated C(sp3)−H bonds remains a desirable and challenging transformation for pharmaceutical, agricultural, and materials scientists. Previous methods for this transformation have used bench-stable fluorine atom sources; however, many still rely on the use of UV-active photocatalysts for the requisite high-energy hydrogen atom abstraction event.
Uranyl nitrate hexahydrate is described as a convenient, earth-abundant, hydrogen atom abstraction catalyst that can mediate fluorinations of certain alkanes upon activation with visible light.