Functionalized Polymer-Supported Pyridine Ligands for Palladium Catalyzed C(sp3)−H Arylation
Li-Chen Lee, Jian He, Jin-Quan Yu, and Christopher W. Jones
ACS Catalysis,
2016, 6, 5245; DOI: 10.1021/acscatal.6b01774
07/2016
Building upon a Center-wide program exploring the immobilization of catalysts for C–H functionalization to enhance the recovery and sustainability of these systems, this report from the Jones and Yu groups describes the latest catalyst system to be added the toolbox of immobilized systems.
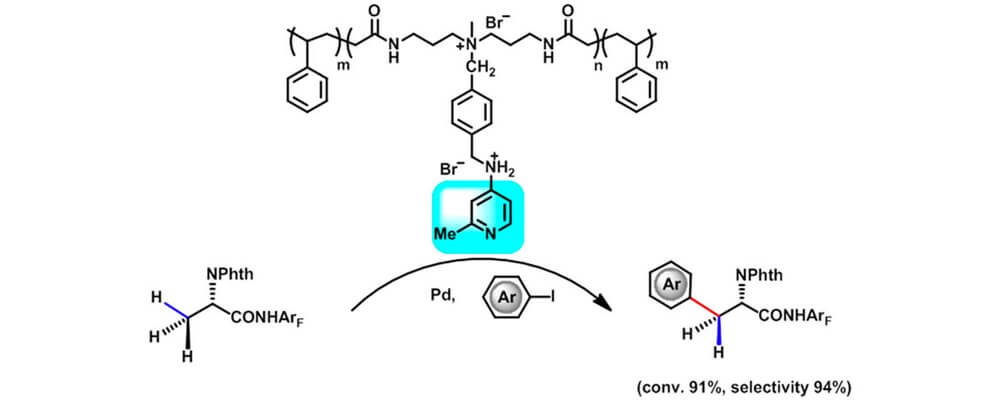
The use of ligands to tune the reactivity and selectivity of transition-metal catalysts for C(sp3)−H bond activation is a current central challenge. One of us previously developed an uncommon example of a homogeneous catalyst that performs controlled C(sp3 )−H arylation using pyridine derivatives as ligands, along with Pd.
In this work, we report a functionalizable and tunable polymer support used in the immobilization of pyridine derivatives that yields a soluble, polymeric ligand platform facilitating C(sp3 )−H activation reactions with good yields, selectivities differing from the homogeneous catalyst, and recovery of Pd. Unlike the homogeneous system, the supported catalysts in Pd-catalyzed C−H monoarylation reactions respond sensitively to the steric hindrance of the coupling partners.
Related Content
-
04/2015
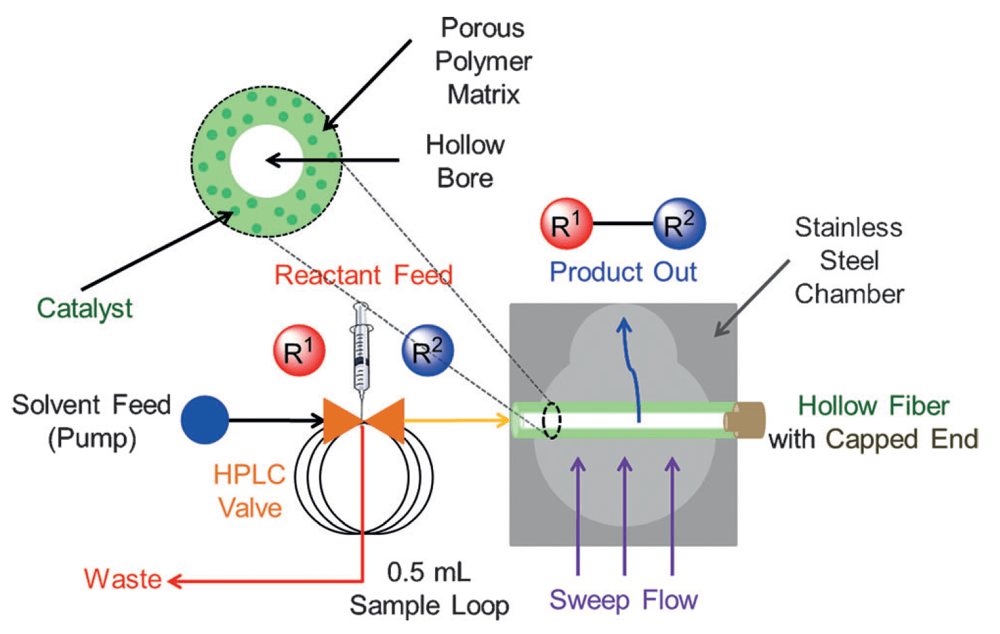
Composite Polymer/Oxide Hollow Fiber Contactors: Versatile and Scalable Flow Reactors
RESEARCH
-
10/2014
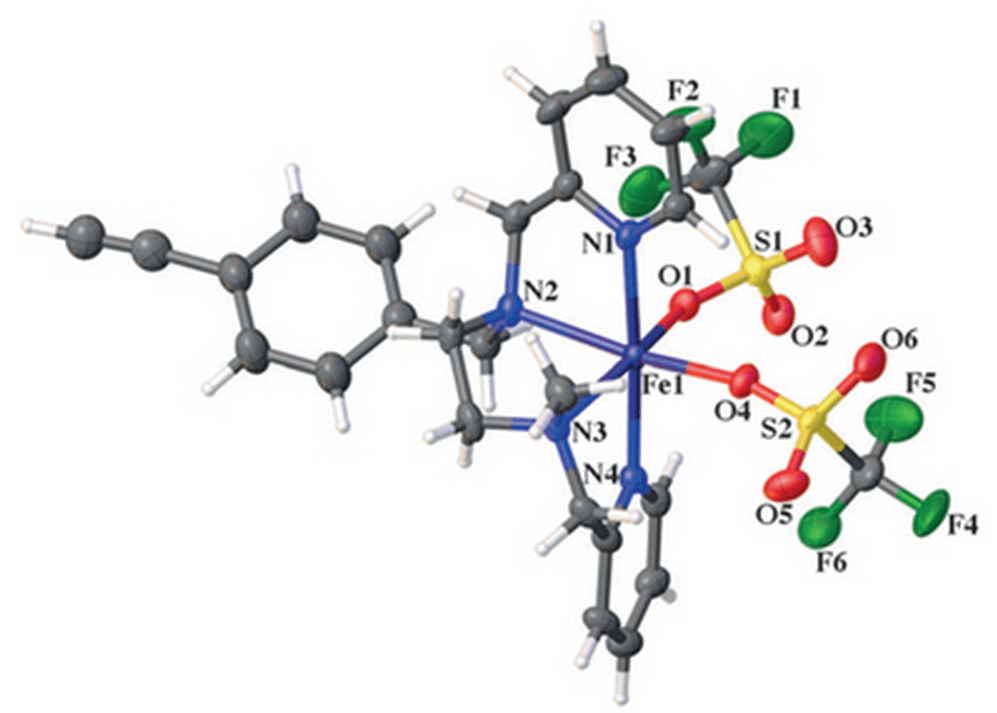
Polymer- and Silica-Supported Iron BPMEN-Inspired Catalysts for C–H Functionalization
RESEARCH
-
04/2014
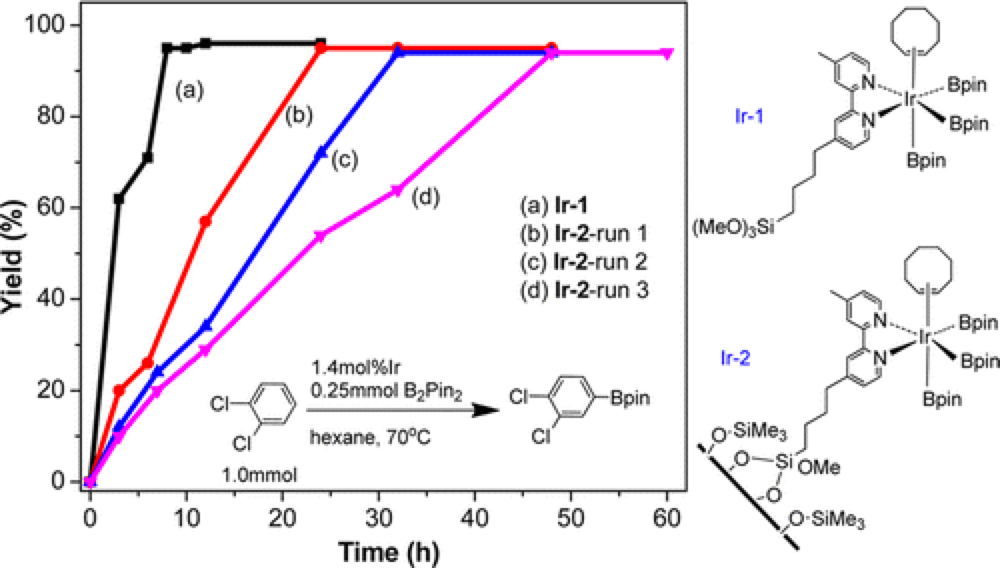
Recyclable Silica-Supported Iridium Bipyridine Catalyst for Aromatic C–H Borylation
RESEARCH