Catalyst-Controlled and Tunable, Chemoselective Silver-Catalyzed Intermolecular Nitrene Transfer: Experimental and Computational Studies
Nicholas Dolan, Ryan J Scamp, Tzuhsiung Yang, John F. Berry, and Jennifer M Schomaker
J. Am. Chem. Soc.,
2016, 138 (44), pp 14658–14667; DOI: 10.1021/jacs.6b07981
10/2016
This collaborative report from the Berry and Schomaker groups describes the development of novel catalysts for selective C–H amination.
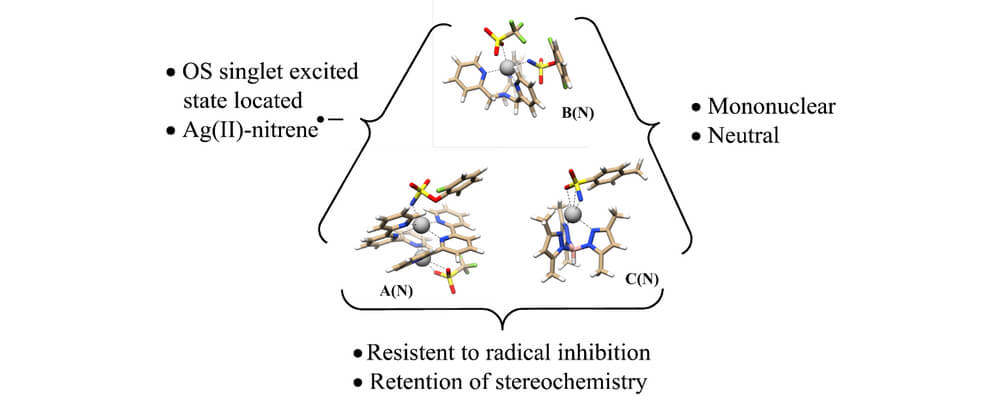
The development of new catalysts for selective nitrene transfer is a continuing area of interest. In particular, the ability to control the chemoselectivity of intermolecular nitrene transfer in the presence of multiple reactive sites has been a long-standing challenge in the field. In this paper, we demonstrate the first examples of silver-catalyzed, non-directed, intermolecular nitrene transfer reactions that are both chemoselective and flexible for aziridination or C–H insertion, depending on the choice of ligand.
Experimental probes present a puzzling picture of the mechanistic details of the pathways mediated by [(tBu3tpy)AgOTf]2 and (tpa)AgOTf. Computational studies elucidate these subtleties and provide guidance for the future development of new catalysts exhibiting tunability in group transfer reactions.