Rh(II)-Catalyzed β-C(sp2)-H Alkylation of Enol Ethers, Enamides and Enecarbamates with α-Diazo Dicarbonyl Compounds
Brett McLarney, Marchello A Cavitt, Djamaladdin G. Musaev, Theodore Donnell, Stefan Anthony France
Chemistry, A European Journal,
2017, 23, 5, 1129-1135; DOI: 10.1002/chem.201604518
11/2016
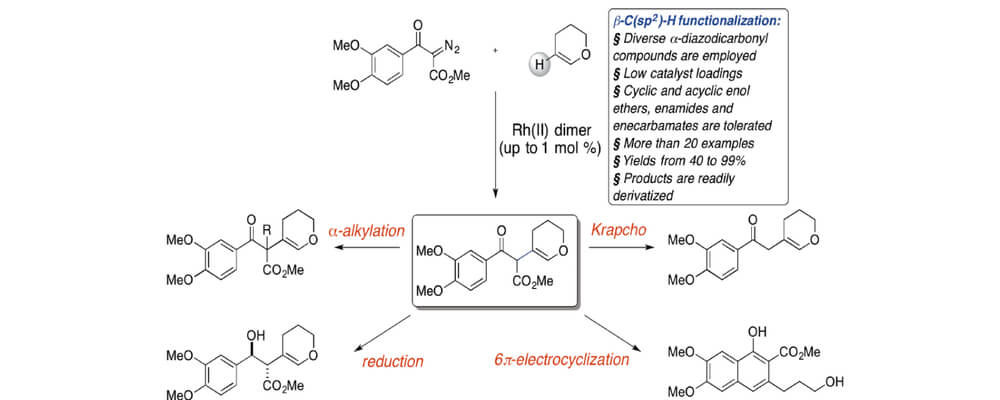
In this collaboration between the France (GA Tech) and Musaev (Emory) groups the dirhodium-catalyzed beta-(Csp2)–H functionalization of enol ethers, enamides and enecarbamates with acceptor/acceptor carbenes is reported. The reaction is highly efficient and the products are demonstrated to be versatile intermediates for further synthetic elaboration.
Using a combination of experimentals and computational studies the presumptive addition-elimination reaction mechanism was explored, findings from which were translated into reaction design, affording the first successful example of the beta-C–H functionalization of acyclic enol ethers using the acceptor carbene systems.
This transformation represents a significant expansion in the scope of substrates for carbene C–H insertion chemistry for future exploitation.