Copper-Catalyzed Bromination of C(sp3)−H Bonds Distal to Functional Groups
Tao Liu, Michael C. Myers, Jin-Quan Yu
Angew. Chem. Int. Ed.,
2017, 129, 1, 312-315; DOI: 10.1002/ange.201608210
11/2016
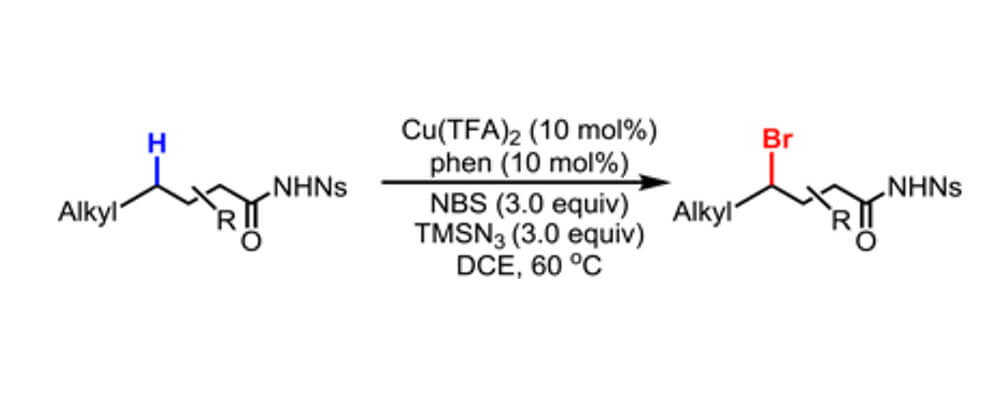
Selective bromination of γ-methylene C(sp3)−H bonds of aliphatic amides and δ-methylene C(sp3)−H bonds of nosyl-protected alkyl amines are developed using NBS as the brominating reagent and catalytic amount of CuII/phenanthroline complexes as the catalyst. Aryl and benzylic C−H bonds at other locations remain intact during this directed radical abstraction reaction.