Toward a mild dehydroformylation using base-metal catalysis
Dylan J. Abrams, Julian G. West and Erik J. Sorensen
Chemical Science,
2017, 8, 1954-1959 ; DOI: 10.1039/C6SC04607J
11/2016
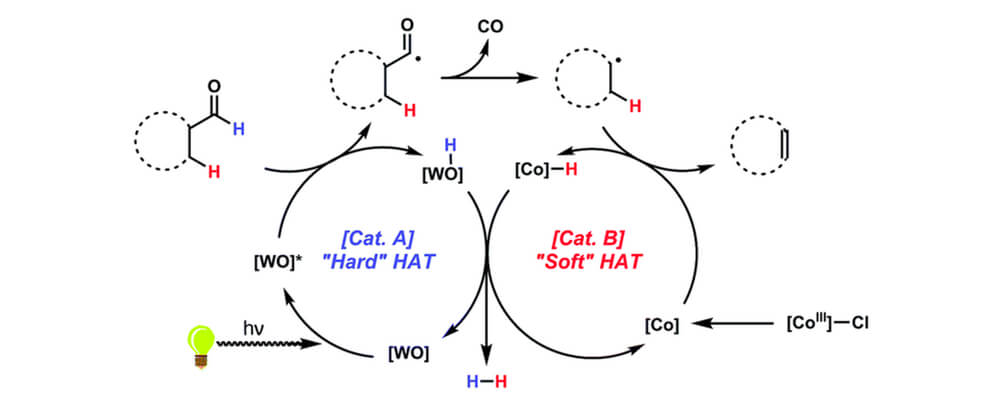
"Hydroformylation, the addition of hydrogen gas and carbon monoxide across an alkene to form an aldehyde product, is an important industrial process for making detergents and other commodity chemicals. Interestingly, the reverse reaction, ""dehydroformylation,"" is almost unknown for chemists, with only a handful of prior examples using expensive noble metals and high temperatures having been discovered. To avoid these drawbacks, we wondered whether a new approach using the cheap, earth-abundant metals tungsten and cobalt could be invented.
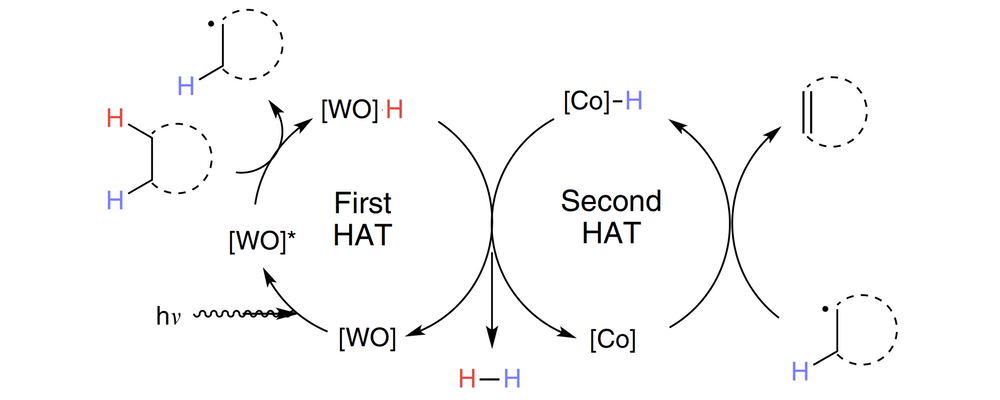
Our reaction takes inspiration from mother nature, whose related ""oxidative demethylation"" relies on a key low-temperature C–H functionalization reaction using the earth abundant element iron. Combining both tungsten and cobalt catalysts and near-ultraviolet light allows for dehydroformylation to be accomplished via the cooperative hydrogen atom transfer mechanism, a process that relies on the two catalysts to each perform a C–H activation reaction. While the reaction is not fully optimized, it represents an important proof-of-concept for designing new catalytic reactions."