β-Carboline Amides as Intrinsic Directing Groups for C(sp2)–H Functionalization
Hélène M.-F. Viart, Andreas Bachmann, William Kayitare, and Richmond Sarpong
J. Am. Chem. Soc.,
2017, 139, (3), pp1325-1329; DOI: 10.1021/jacs.6b12569
01/2017
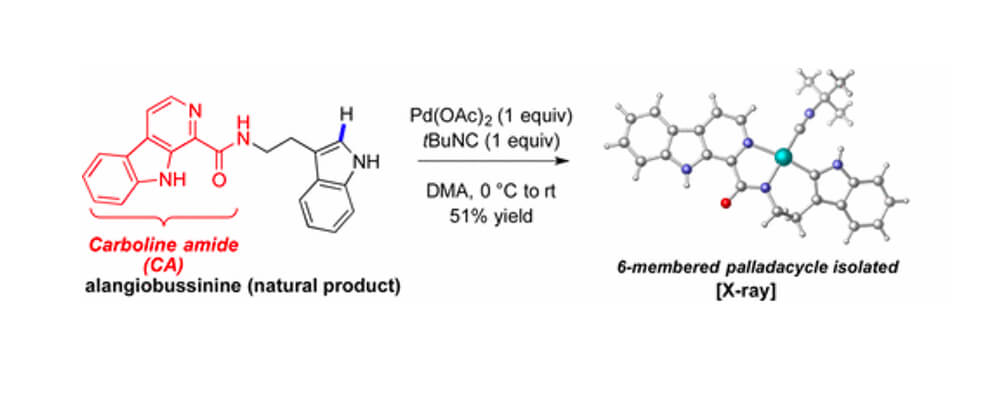
Many site-selective palladium-catalyzed C–H functionalization methods require directing groups. We report here β-carboline amides as intrinsic directing groups for C(sp2)–H functionalization.
Various substrates including the natural product alangiobussinine and the marinacarboline core structure were functionalized using carboline-directed δ-C(sp2)–H alkynylations. This transformation proceeds under mild conditions and is compatible with a wide variety of β-arylethamines.
δ-Alkynylation of β-arylethamines via a six-membered palladacycle is favored over γ-C(sp2)–H bond functionalization when both positions are accessible. The versatility of β-carboline amides as directing groups is evidenced by other δ-C(sp2)–H functionalizations such as alkenylation, arylation, and C–N bond formation.