Synthesis of 2,2,2,-Trichloroethyl Aryl- and Vinyldiazoacetates by Palladium-Catalyzed Cross-Coupling
Liangbing Fu, Jeffrey Mighion, Eric Voight, Huw Davies
Chem. Eur. J.,
2017, 23, 14, 3272-3275; DOI: 10.1002/chem.201700101
01/2017
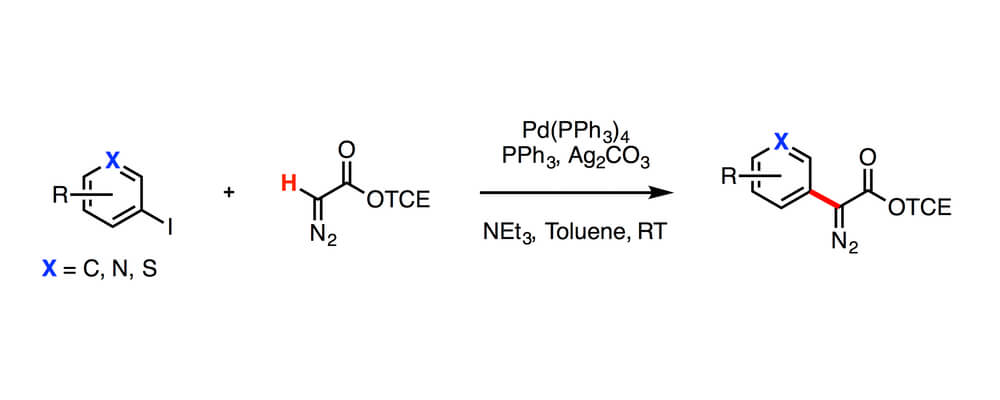
Diazo compounds are important precursors for the generation of carbenes used in C–H insertion reactions, however the synthesis of them can often involve difficult reagents and is limited in scope. In this report a Pd-catalyzed C–H functionalization approach fot the streamlined synthesis of diazo compounds is disclosed, that increases the scope.
An efficient and convenient synthesis of 2,2,2-trichloroethyl (TCE) aryl- and vinyldiazoacetates was achieved by palladium-catalyzed cross-coupling reactions between TCE diazoacetates and aryl or vinyl iodides. The broad substrate scope allows for rapid and facile formation of TCE aryl- and vinyl-diazoacetates, which recently have emerged as versatile reagents for rhodium-carbene chemistry.