Metal-Free C–H Functionalization of Alkanes by Aryldiazoacetates
Cecilia Tortoreto, Daniel Rackl, and Huw M. L. Davies
Organic Letters,
2017, 19, (4), 770-773; DOI: 10.1021/acs.orglett.6b03681
02/2017
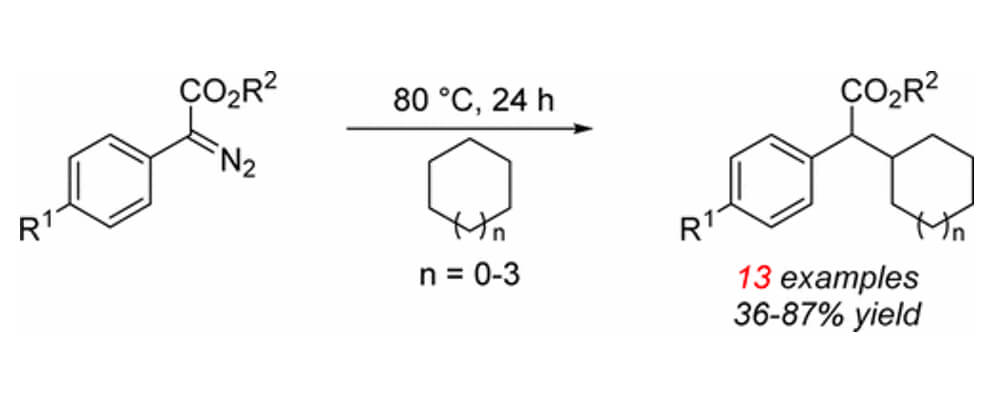
Thermally induced reactions of donor/acceptor diazo compounds generate carbene intermediates capable of C–H functionalization reactions of alkanes. A variety of C–H insertion products were obtained in moderate to good yields and in certain cases with good site selectivity, favoring the functionalization of the more highly substituted C–H bond.
This report from the Davies group effectively sets a baseline for the reactivity and selectivity of thermally induced carbenes, against which catalyst controlled reactions can be benchmarked.