2-Br PDI: synthesis using C–H activation and use in the synthesis of bis(perylene diimide)–donor electron-transport materials
Junxiang Zhang, Sanjeev Singh, Do Kyung Hwang, Stephen Barlow, Bernard Kippelen and Seth R. Marder
J. Mat. Chem. C.,
2013, 1, 5093-5100; 10.1039/C3TC30918E
07/2013
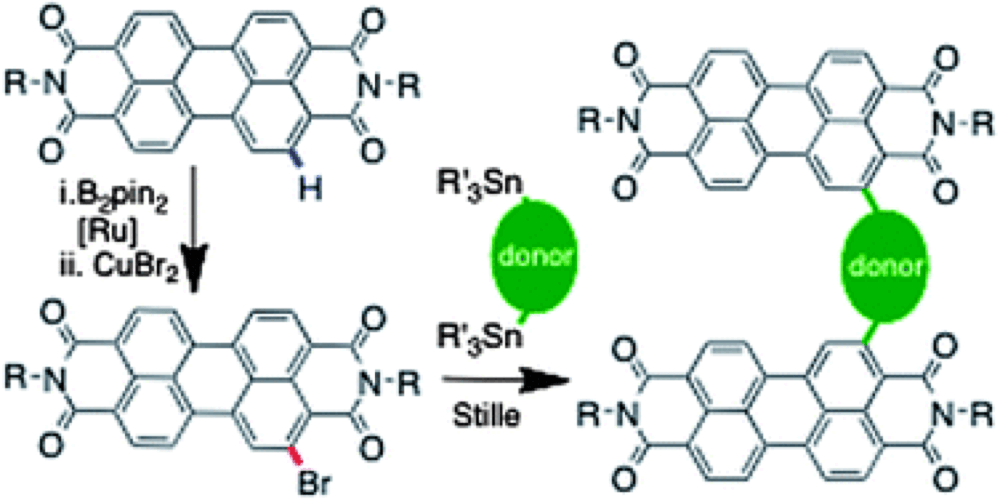
Perylene Diimides (PDI's) are key building blocks for the construction of organic field-effect transistors (OFET's), due to their high electron affinities and mobilities. More traditional electrophillic halogenation reactions occur at the 1,6,7 and 12 positions due to the strongly meta-directing character of the imide groups, however substitution or bridging at these positions have been found to result in significant twisting of the poly-PDI's, which disrupt the pi-pi stacking of these oligiomers and are presumably detrimental to electron mobility.
Metal catalyzed C-H activation has opened up a new method to functionalize the 2,5,8 and 11 positions and hence explore the differently substituted oligiomers that are predicted to have less of a twisted nature. This report by the Marder group describes synthesis of such oligiomers and investigation of their properties through C-H Activation.