The Origins of Dramatic Differences in Five-Membered vs Six-Membered Chelation of Pd(II) on Efficiency of C(sp3)–H Bond Activation
Yun-Fang Yang, Gang Chen, Xin Hong, Jin-Quan Yu, and K. N. Houk
J. Am. Chem. Soc.,
2017, 139, (25), 8514-8521; DOI:10.1021/jacs.7b01801
06/2017
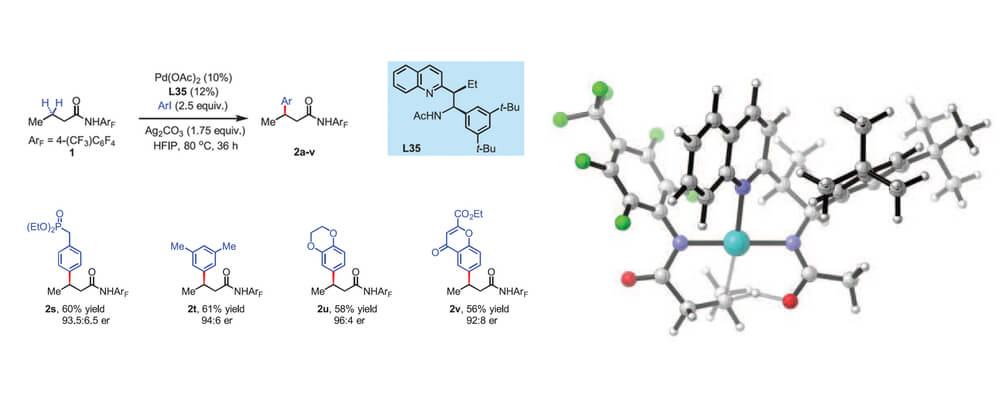
The origin of the unique effectiveness of six-membered chelates on the β-methylene C(sp3)–H activation reactions by Pd(II) catalyst was explained with density functional theory. The Pd(II) catalysts that involve five-membered chelates are inactive in this transformation. Computational studies suggest that the C(sp3)–H bond activation is the rate-limiting step in both cases.
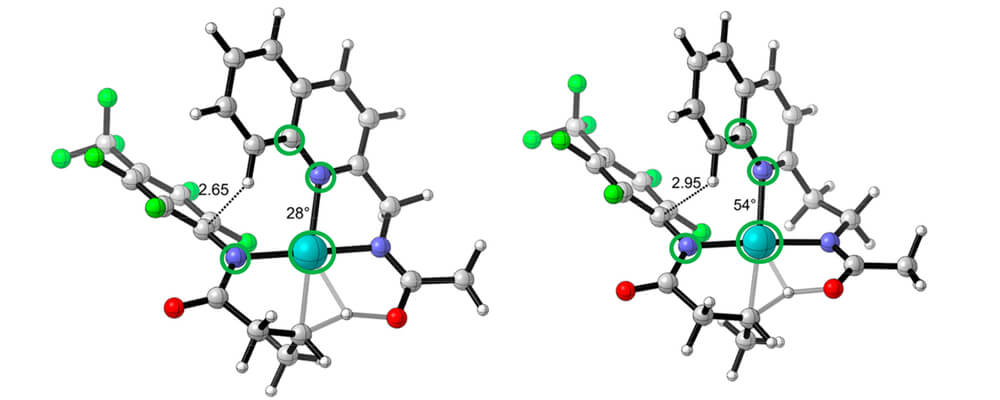
The C(sp3)–H bond activation with a five-membered chelate is unfavorable by 7.7 kcal/mol compared to the corresponding six-membered chelate with Pd(II). Two factors cause the difference: (1) the dimeric Pd species with five-membered chelation square-planar structure is more stable than that with six-membered chelation by 2.0 kcal/mol; (2) steric repulsion between the ArF group of the substrate and the quinoline group of the acetyl-protected aminomethyl quinoline ligand destabilizes the five-membered chelate transition structure by 5.7 kcal/mol.
The six-membered chelate of Pd(II) with an acetyl-protected aminoethyl quinoline ligand orients the ligand away from the ArF group of the substrate and alleviates the steric repulsion.