Enantioselectivity Model for Pd-Catalyzed C–H Functionalization Mediated by the Mono-N-protected Amino Acid (MPAA) Family of Ligands
Brandon E. Haines, Jin-Quan Yu, and Djamaladdin G. Musaev
ACS Catalysis,
2017, 7, (7), 4344-4354; DOI:10.1021/acscatal.7b01281
05/2017
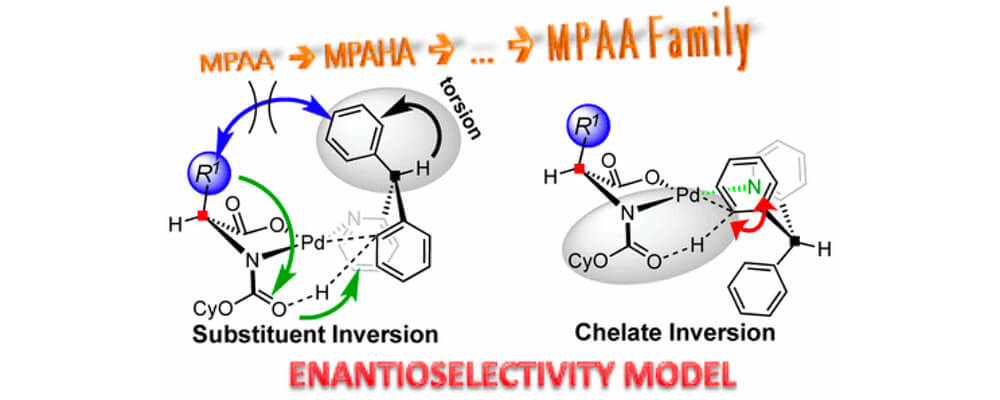
The mono-N-protected amino acid (MPAA) family of ligands is arguably the most prolific scaffold for enabling enantioselective C–H activation via metal insertion. However, its mechanism for asymmetric induction is not fully understood. In this collaborative work between the Musaev Groups, a practical model for asymmetric induction from the viewpoint of rationalizing chiral, bidentate ligands for C–H activation via metal insertion is described.
The model is validated against Pd(II)-catalyzed C–H activation in 2-benzhydrylpyridine mediated by the [(cyclohexyloxy)carbonyl]-l-leucine (MPAA) ligand. We found that full control elements of enantioselectivity are (a) inherent strain in the bidentate coordination of substrate and the ability of the ligand to enhance it, (b) the gearing effect that restricts the C–H bond activation to one face of the Pd catalyst, (c) potential interactions between the ligand and substrate, and (d) chelate inversion which is controlled by the ligand-anchored base that restricts the C–H bond activation to one side of the Pd catalyst.