Palladium-Catalyzed Transformations of Alkyl C–H Bonds
Jian He, Masayuki Wasa, Kelvin S. L. Chan, Qian Shao and Jin-Quan Yu
Chemical Reviews,
2017, 117, (13), 8754; DOI:10.1021/acs.chemrev.6b00622
01/2017
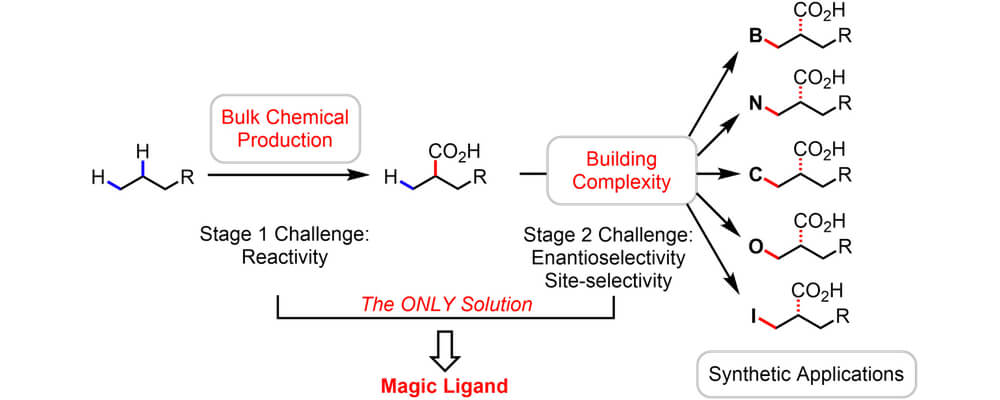
Palladium-catalyzed C–H activation has emerged as one of the most effective technologies in the field of C–H functionalization. However, there are a number of restrictions associated with this chemistry, including the reliance on directing groups and the scope limitation to primarily sp and sp2 C–H bonds.
This review from the Yu group examines the recent advances in palladium-catalyzed C(sp3)–H bond activation. There have been only a few examples of the activation of simple alkane C–H bonds, but recent developments in understanding and catalyst design have driven the discovery of reactions capable of effectively exploiting the presence of a directing group to functionalize C(sp3)–H bonds. Furthermore, a number of mono- ad bidentate ligands have been demonstrated to accelerate C(sp3)–H activation directed by weakly coordinating auxiliaries, which reveal exciting possibilities to control reactivity, selectivity and stereoselectivity in palladium-catalyzed C–H functionalization reactions.