Pomeranz–Fritsch Synthesis of Isoquinoline: Gas-Phase Collisional Activation Opens Additional Reaction Pathways
Shibdas Banerjee, Fang Liu, David M. Sanchez, Todd J. Martínez, and Richard N. Zare
J. Am. Chem. Soc.,
2017, 139. (41), 14352-14355; DOI:10.1021/jacs.7b06813
09/2017
This collaborative report from the Zare and Martinez groups, both based at Stanford, describes a detailed study investigating some unusual observations on a classic reaction.
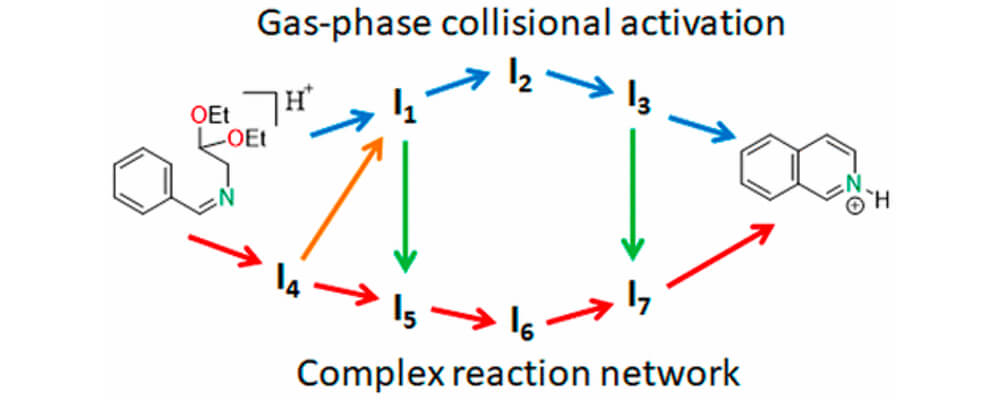
The Pomeranz-Fritsch reaction, first reported in 1893, provides rapid access to isoquinolines, a molecular motif found throughout pharmaceutical and materials chemistry. A recent observation from the Zare group showed that this reaction could be accelerated by nearly a million times by performing the reaction in charged microdroplets, instead of the traditional bulk liquid phase. This is important not only because of the speed and ease of the reaction, but also because the liquid phase requires the use of concentrated strong acids, which is not required in the charged microdroplet system.
This study that brings together a suite of analytical and theoretical tools, include mass spectrometry, collisional activation and theoretical calculations, takes a deep dive into the mechanism of this reaction, finding the while the starting materials and products of this reaction in both the liquid and charged gaseous phase are the same, the reaction mechanism is quite different. The proton mobility in the gaseous activated states allows passage through a number of intermediates which cannot be formed in solution phase.