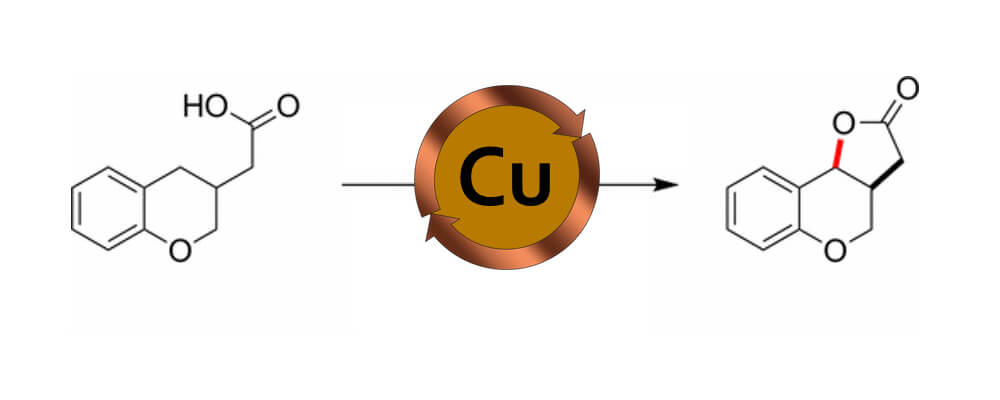
Mechanistic analysis of a copper-catalyzed C–H oxidative cyclization of carboxylic acids
Shibdas Banerjee, Shyam Sathyamoorthi, J. Du Bois and Richard N. Zare
Chemical Science,
2017, 8, 7003-7008; DOI:10.1039/C7SC02240A
08/2017
This collaborative report from the Du Bois and Zare labs describes the investigation of a copper catalyst capable of C–H oxidative cyclization of carboxlyic acids.
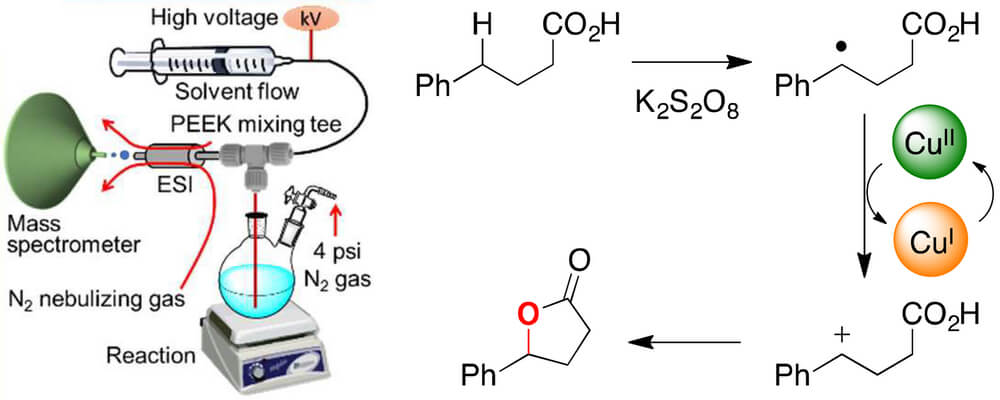
We recently reported that carboxylic acids can be oxidized to lactone products by potassium persulfate and catalytic copper acetate. Here, we unravel the mechanism for this C–H functionalization reaction using desorption electrospray ionization, online electrospray ionization, and tandem mass spectrometry. Our findings suggest that electron transfer from a transient benzylic radical intermediate reduces Cu(II) to Cu(I), which is then re-oxidized to Cu(II) in the catalytic cycle. The resulting benzylic carbocation is trapped by the pendant carboxylate group to give the lactone product.
Formation of the putative benzylic carbocation is supported by Hammett analysis. The proposed mechanism for this copper-catalyzed oxidative cyclization process differs from earlier reports of analogous reactions, which posit a substrate carboxylate radical as the reactive oxidant.