Dynamic Ligand Exchange as a Mechanistic Probe in Pd-Catalyzed Enantioselective C–H Functionalization Reactions Using Monoprotected Amino Acid Ligands
David E. Hill, Katherine L. Bay, Yun-Fang Yang, R. Erik Plata, Ryosuke Takise, K. N. Houk, Jin-Quan Yu, and Donna G. Blackmond
J. Am. Chem. Soc.,
2017, 139, (51), 18500; DOI:10.1021/jacs.7b11962
12/2017
Catalysts are tools developed by chemists that expediate the production of materials and energy used in daily life. Catalysts serve this important role by providing access to a lower energy reaction pathway that was previously unavailable, thereby decreasing the overall energy required for the chemical transformation. Understanding the way current catalysts work, or their mechanism, is critical to their advancement and diversification. Thus, research in advancing and understanding catalysts has led to the development of various methods for interrogating catalyst mechanisms. One such method is chemical kinetics, or the study of reaction rates, which is the primary tool employed by the Blackmond lab to investigate catalyst mechanisms. Through observing reaction rates under different conditions, the Blackmond lab builds experimentally-derived mathematical models that plausibly describe the individual steps of an entire catalyst mechanism. Previous investigations by the Blackmond lab have been influential towards validating or disproving the accepted hypotheses for a catalyst’s mechanism. The research described below illustrates the Blackmond lab’s work to characterize a previously unknown mechanistic feature of a C-H/iodination catalyst.
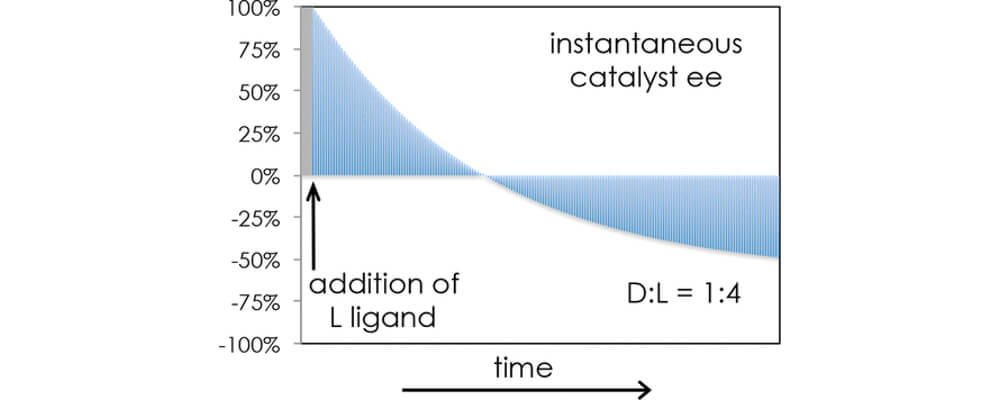
Catalysts are often composed of two components, a metal center and ligands, groups of molecules that surround the metal center. Previous mechanistic studies on this chiral C-H/iodination catalyst by the Blackmond lab revealed that the initial ligands on the catalyst did not remain in place over the duration of the reaction. Other ligands present in the reaction mixture could replace the original ligands on the catalyst. These ligand substitution processes were previously known for other catalysts; however, there were limited studies and methods for quantifying ligand exchange on a catalyst during a chemical reaction. The Blackmond lab was intrigued by these initial ligand exchange findings for the C-H/iodination catalyst and developed an experimental technique that would monitor ligand exchange during the chemical reaction.
Using this newly developed technique, the Blackmond lab found the rate of ligand exchange on the catalyst was significantly faster than the rate of the chemical reaction. Although this new technique could measure the rate of ligand exchange during the reaction, additional experiments were not able to distinguish between multiple plausible ligand exchange mechanisms. For this reason, the Blackmond lab collaborated with the Houk lab, a group specializing in computational chemistry, to model the energy required for each ligand exchange pathway. Through combining the computer modeling by the Houk lab and experimental measurements from the Blackmond lab, the most plausible ligand exchange mechanism and the relative rate of ligand exchange to rate of chemical reaction were determined. This new method for monitoring ligand exchange on a catalyst during a reaction provides chemists access to a mechanistic feature that was previously unavailable through other techniques. Understanding the dynamics of ligands during a reaction will aid in the advancement and diversification of catalysts.
Author: David Hill