Selective Intermolecular Amination of C-H Bonds at Tertiary Carbon Centers
Jennifer L. Roizen, David N. Zalatan and Justin Du Bois
Angew. Chem. Int. Ed.,
2013, 52, 11343-11346; 10.1002/anie.201304238
09/2013
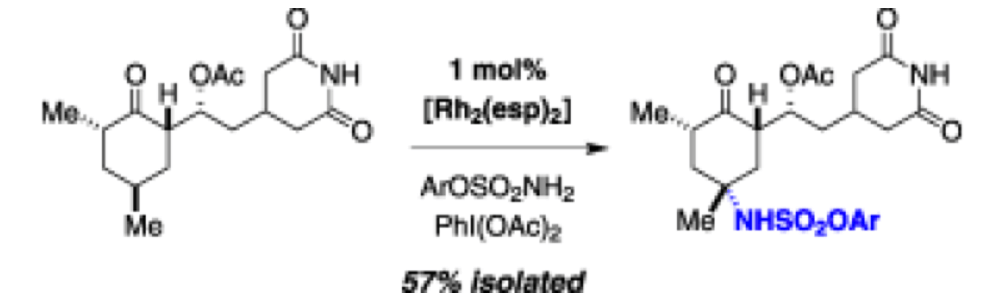
A method for intermolecular amination of tertiary C–H bonds is described that uses limiting amounts of substrate and a convenient phenol-derived nitrogen source. Structure-selectivity and mechanistic studies suggest that steric interaction between the substrate and active oxidant is the principal determinant of product selectivity.