Understanding and Improving the Activity of Flavin-Dependent Halogenases via Random and Targeted Mutagenesis
Mary C. Andorfer and Jared C. Lewis
Ann. Rev. Biochem.
2018, 87, 159-185; DOI:10.1146/annurev-biochem-062917-012042
03/2018
One of the key drives within the Center’s development of catalysts capable of selectively functionalizing C–H bonds in a reliable and predictable fashion is the understanding of how Nature’s catalysts, enzymes, perform this reaction, and beyond that, how we can modify these enzymes to perform synthetically useful transformations.
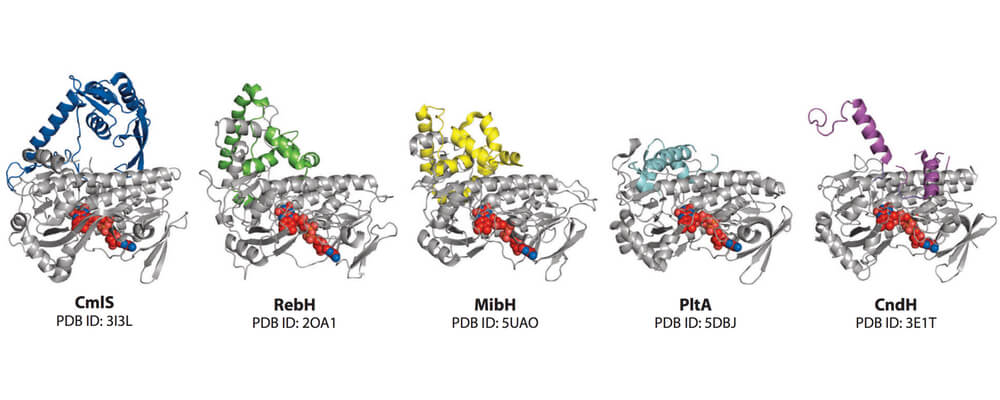
This review from the Lewis group summarizes recent investigations of Flavin-dependent Halogenases (FDH), a class of enzyme that catalyze the halogenation of organic substrates through the coordination of reactions of reduced flavin, molecular oxygen and chloride.
Targeted and random mutagenesis approaches have been used to better understand and alter their reactivity, providing insights into the roles of specific residues in the protein, leading to the development of FDH variants with improved stability, expanded substrate scope and altered site selectivity.
Through the development of these FDH mutants it has become clear that much reactivity remains to be discovered within this enzyme class. Future efforts, including FDH genome mining will help uncover these abilities. The understanding and knowledge gained from the studies of this class of enzymes are expected to broadly inform studies on other enzyme classes.