Regioselective Intermolecular Allylic C−H Amination of Disubstituted Olefins via Rhodium/π‐Allyl Intermediates
Jacob S. Burman and Simon B. Blakey
Angew. Chem. Int. Ed.,
2017, 56, 44, 13666-13669; DOI: 10.1002/anie.201707021
09/2017
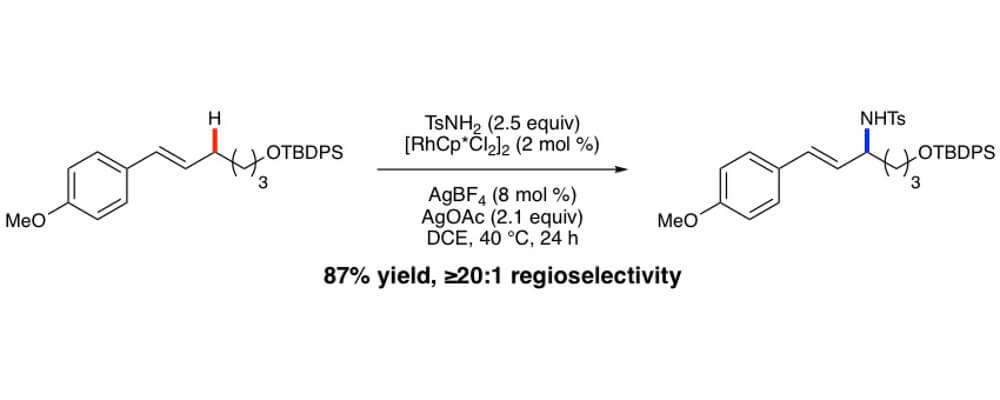
The Blakey Group reports a method for the catalytic intermolecular allylic C–H amination of trans-disubstituted olefins. The reaction is efficient for a range of common nitrogen nucleophiles bearing a single electron withdrawing group and proceeds under mild reaction conditions. Good to excellent levels of regioselectivity are observed for a wide range of electronically diverse trans-ß-alkyl styrene substrates. Reactivity and selectivity of the allylic amination is not hindered with substrates bearing a 2° or 3° homo-allylic carbon. However, substrates bearing a 4° homo-allylic carbon do exhibit lower yields and regiochemistry from the transformation. Initial mechanistic investigations revealed an equilibration of observed products toward the conjugated regioisomer at elevated temperatures and extended reaction time.