Synthetic Studies Toward Pactamycin Highlighting Oxidative C–H and Alkene Amination Technologies
Justin Y. Su, David E. Olson, Stephen I. Ting, and Justin Du Bois
J. Org. Chem.,
2018, 83, (13), 7121–7134; DOI: 10.1021/acs.joc.8b00142
04/2018
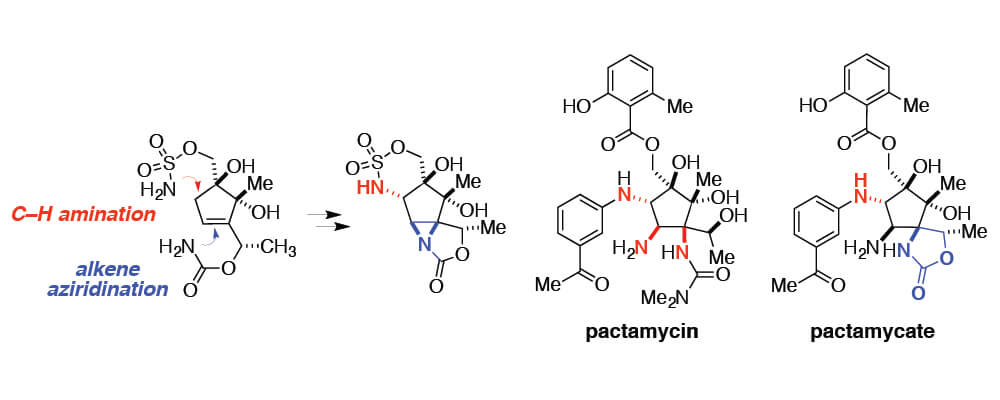
A strategy featuring the application of contemporary methods for the selective oxidation of C–H and -bonds is outlined for the synthesis of pactamycin. Chemoselective oxidative cyclization of an intermediate sulfamate introduces the first of three N-centers. By employing this technology, the core of C2-epi-pactamycate has been successfully assembled. The many challenges associated with the synthesis of pactamycin and related aminocyclitol natural products will continue to drive innovation in oxidation catalysis.