Pd-Catalyzed, ortho C–H Methylation and Fluorination of Benzaldehydes Using Orthanilic Acids as Transient Directing Groups
Xiao-Yang Chen and Erik J. Sorensen
J. Am. Chem. Soc.,
2018, 140 (8), pp 2789–2792; DOI: 10.1021/jacs.8b00048
02/2018
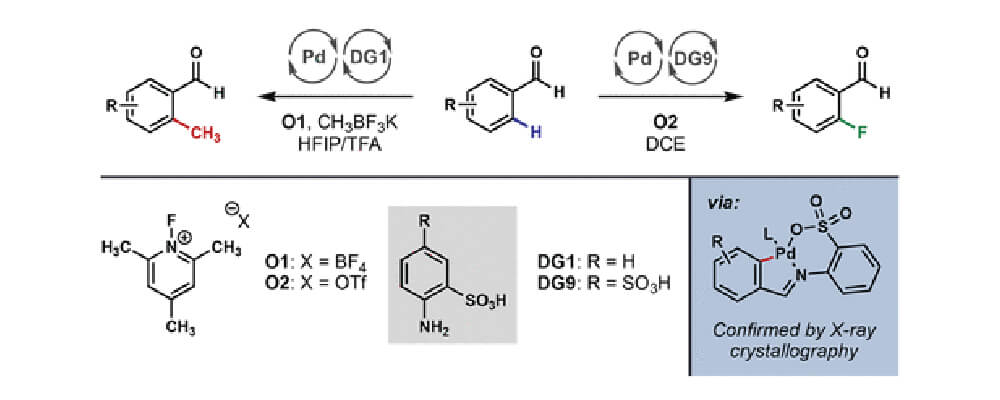
While broadly regarded as privileged pharmacophores due to their remarkable ability to improve the pharmacological properties of biologically active compounds, C-CH3 and C-F bonds can be notoriously difficult to construct from C-H bonds. Herein, a catalytic method was developed for the direct ortho C-H methylation and fluorination of benzaldehydes via Pd catalysis.
Central to these transformations was the use of a new type of TDGs, orthanilic acid, which contains sulfonic acid as the X-type ligand. Compared to the traditional anthranilic acid, which has a carboxylic acid group as the X-type ligand, this new TDG exhibited unprecedented reactivity in C-H methylation and fluorination reactions. By fine tuning of the F+ oxidants, the TDGs, and the solvents, good yields and functional group compatibility could be achieved for both reactions.
A single X-ray crystal structure of the benzaldehyde ortho C-H palladacycle was also obtained by trapping the insertion intermediate with triphenylphosphine.