Unveiling the Role of Base and Additive in the Ullmann-Type of Arene-Aryl C–C Coupling Reaction
Manjaly J. Ajitha, Fathima Pary, Toby L. Nelson, and Djamaladdin G. Musaev
ACS Catalysis,
2018, 8 (6), pp 4829–4837; DOI: 10.1021/acscatal.8b00837
04/2018
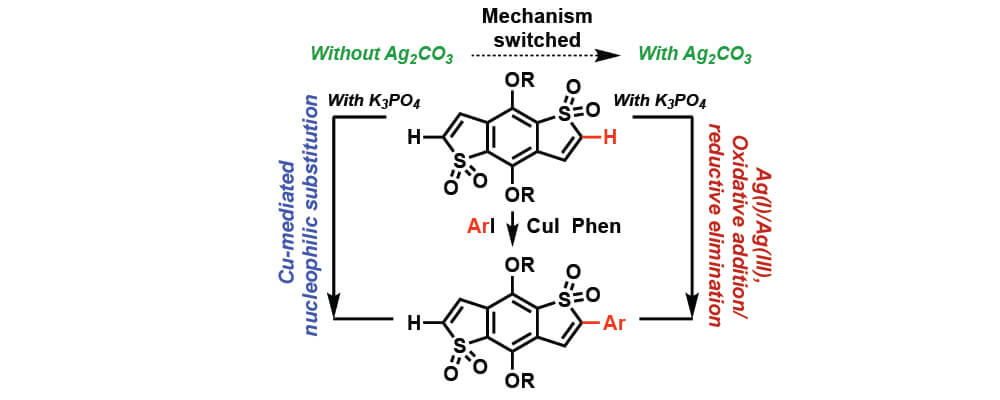
The mechanism of Ullmann-type biaryl formation between benzo-[1,2-b:4,5-b’]dithiophene-1,1,5,5-tetraoxide (BDTT) and iodobenzene (ArI) was computationally studied in the presence of CuI, phenanthroline (Phen), K3PO4 (as a base) and Ag2CO3 (as an additive). It is also shown that base and additive play critical roles in each steps of the reaction. It is shown that in the presence of K3PO4 and in the absence of additive Ag2CO3, the Ph-I activation and C-C coupling, occurs via a Cu-mediated nucleophilic substitution mechanism, and requires a significant free energy barrier.
However, the addition of Ag2CO3 to the reaction mixture, not only accelerates the DBT and PhI coupling by reducing the rate-limiting Ph-I activation barrier, but also switches the mechanism of the reaction from a Cu-mediated nucleophilic substitution to a Ag(I)-promoted oxidative addition-reductive elimination.