Non-Directed Allylic C–H Acetoxylation in the Presence of Lewis Basic Heterocycles
Hasnain A. Malik, Buck L. H. Taylor, John R. Kerrigan, Jonathan E. Grob, Ken Houk, Justin Du Bois, Lawrence Hamann and Andrew W. Patterson
Chemical Science,
2014, 5, 2352-2361; 10.1039/C3SC53414F
02/2014
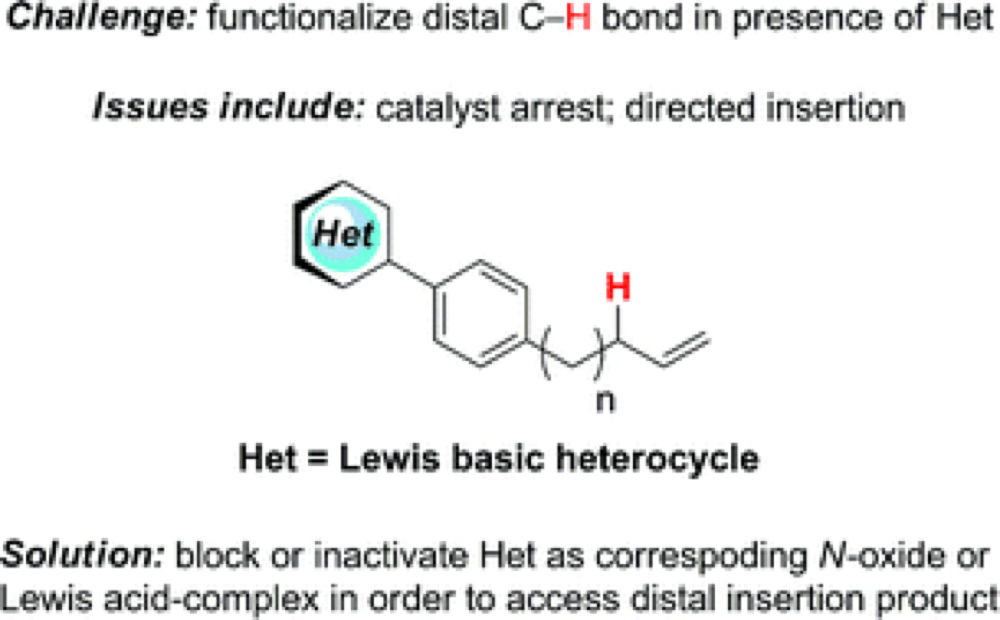
One of the limitations encountered in the application of C–H Functionalization across a broad scope of chemical targets is the interaction of the highly reactive reagents required to functionalize a C–H bond and Lewis Basic functionalities in substrates. The labile electron lone-pair of these functionalities interacts readily with the reagents, either reacting directly or directing the reaction to an undesired location.
A typical strategy to overcome this has been to protect functionalities such as amines and alcohols; however, this is not always amenable to streamlined synthesis or particular strategies.
Pharmaceutical therapeutic targets often incorporate many Lewis Basic sites, it is one of the key ways in which medications interact with sites in the body, thus the adaptation and development of C–H Functionalization transformations in the presence of Lewis Basic sites is a central focus of the CCHF’s collaboration with our pharmaceutical industrial partners, Novartis.
This report, which brings together the Dubois, Houk and Novartis groups from the CCHF takes this challenge head on, developing and validating a rapid and effective screening method for the efficacy of new methods in the presence of Lewis Basic sites, but also demonstrating two new masking techniques which allow the C–H hydroxylation of allylic C–H bonds.
This works lays the foundation for the rigorous testing of new, and more established, techniques against substrates and conditions applicable to pharmaceutically relevant targets.