Manganese terpyridine artificial metalloenzymes for benzylic oxygenation and olefin epoxidation
Chen Zhang, Poonam Srivastava, Ken Ellis-Guardiola, Jared C. Lewis
Tetrahedron,
2014, 70, 4245-4249; 10.1016/j.tet.2014.03.008
07/2014
The Lewis group from the University of Chicago report a novel artificial enzyme hybrid that is effective in the oxygenation of benzylic C–H bonds and epoxidation of olefins.
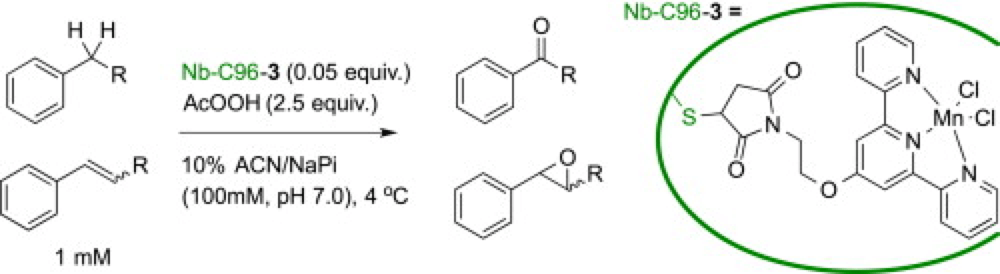
By incorporating a synthetically derived manganese-terpyridine co factor into a protein scaffold an artificial metalloenzyme (ArM) was created. The idea is that the specific three-dimensional shape and orientation of the protein, that imparts such exquisite selectivity in nature, could be used to control the selectivity and reactivity of the synthetic co-factor.
After screening a number of different protein scaffold Nb-C96 was chosen as it showed the highest levels of co-factor incorporation. After some optimization of the conditions this ArM was found to be highly effective at oxidizing benzylic C–H bonds and epoxidizing olefins across a broad scope of substrates.
This exciting new class of catalyst that employs Natures scaffolds to impart selectivity upon previously unselective reagents has huge potential in the development of a suite of catalysts for C–H functionalization.