C7-Derivatization of C3-Alkylindoles Including Tryptophans and Tryptamines
Richard P. Loach, Owen S. Fenton, Kazuma Amaike, Dustin S. Siegel, Erhan Ozkal, and Mohammad Movassaghi
J. Org. Chem.,
2014, 79, (22), 11254-11263; 10.1021/jo502062z
11/2014
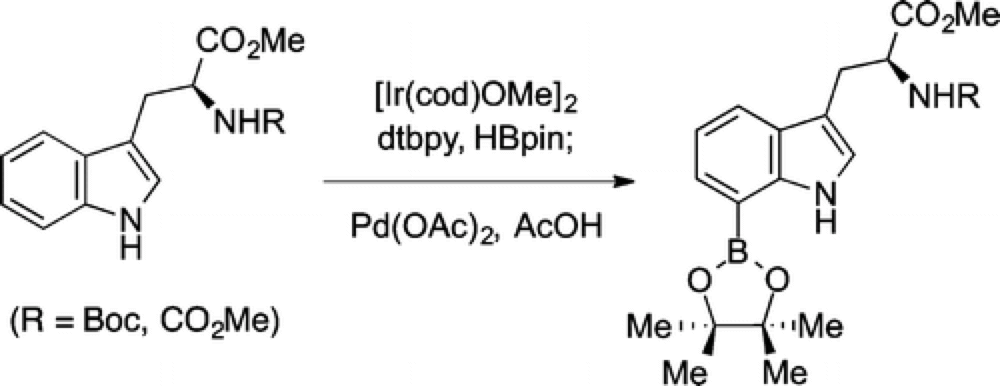
The Movassaghi group at MIT reports an expedient method for the derivatization of C3-alkylindoles at the C7 position.
Introducing substituents at this position is necessary for the synthesis of a number of classes of natural product alkaloids, including the teleocidins and biologically active molecules.
Previous methods developed to introduce this functionality in a scalable fashion required multiple complex operations, significantly reducing the efficiency of the strategy and starting from simple aryl systems and building up the indole motif in a stepwise method.
Employing C–H Functionalization the Movassaghi group have developed a one-pot, two-step process, applicable to a wide range of indoles, proceeding in good yield on a practical scale.
Kazuma Amaike is a graduate student from the Itami group from Nagoya University in Japan. Kazuma was visiting the Movassaghi lab as part of the international network that the CCHF is part of, the VICHF.
The transformation proceeds by iridium-catalyzed boronation, which reacts at both the C2 and C7 positions, followed by in-situ palladium-catalyzed selective protodeboronation at the C2 position.
A further advantage of this strategy is that the C7-boronated products can be readily transformed into a diverse range of substituted indoles.