2,2,2-Trichloroethyl Aryldiazoacetates as Robust Reagents for the Enantioselective C–H Functionalization of Methyl Ethers
David M. Guptill and Huw M. L. Davies
J. Am. Chem. Soc.,
2014, 136, (51), 17718-17721; 10.1021/ja5107404
12/2014
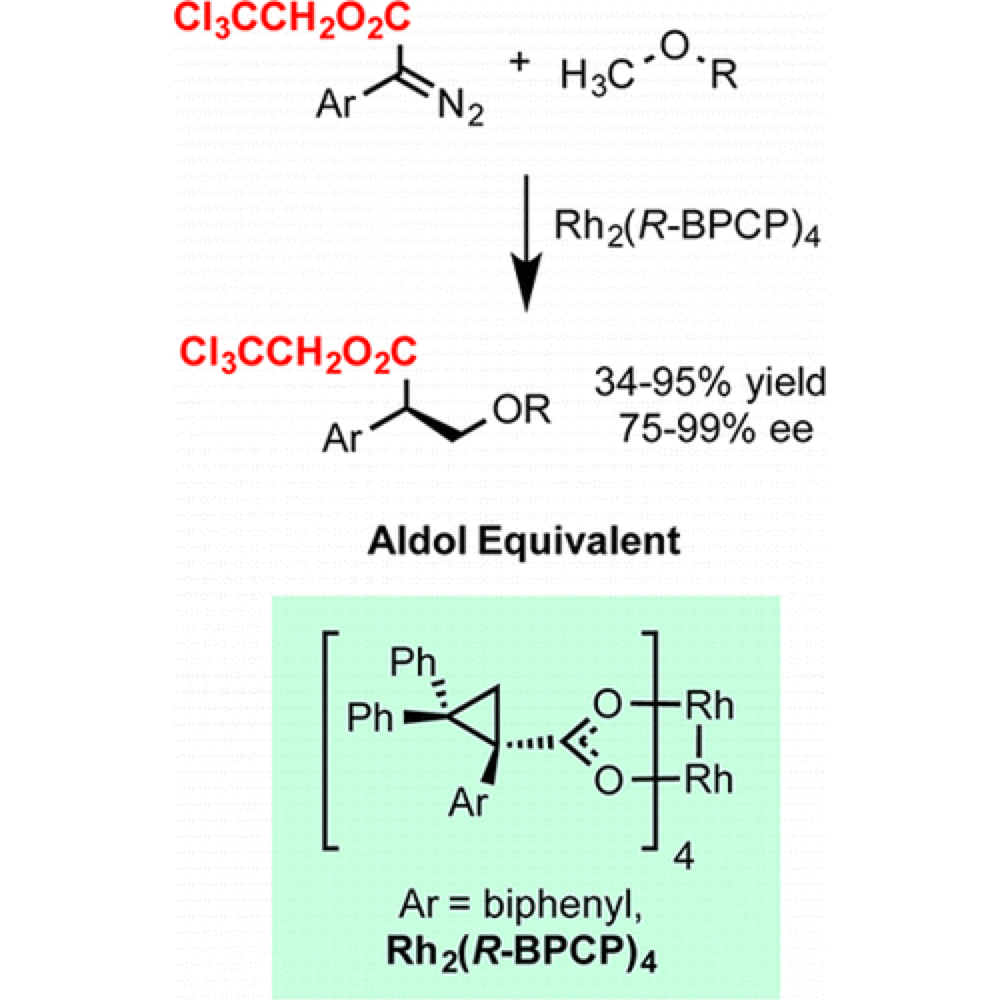
The Davies group have developed a new class of robust carbene precursors that enable superior levels of reactivity and selectivity in intermolecular C–H insertion.
These 2,2,2-trichloroethyl aryl and heteroaryl donor/acceptor diazoacetates are not susceptible to intramolecular side reactions and demonstrate a lower propensity to dimerization of the carbenes. This improved reaction profile has allowed intermolecular C–H insertion with substrates previously impossible.
With this new reaction profile previously challenging substrate can be employed to provide access to new products and provides the potential for new reaction and reactor systems.