A Combined IM-MS/DFT Study on [Pd(MPAA)]-Catalyzed Enantioselective C–H Activation: Relay of Chirality through a Rigid Framework
Gui-Juan Cheng, Ping Chen, Tian-Yu Sun, Xinhao Zhang, Jin-Quan Yu, and Yun-Dong Wu
Chem. Eur. J.
2015, 21, 31, 11180–11188; 10.1002/chem.201501123
06/2015
Chiral mono-protected amino acids (MPAA’s) have been established as privileged and powerful ligands in enantioselective Pd-catalyzed C–H activation chemistry, capable of efficient activation of both C(sp2)–H and C(sp3)–H bonds. Though the subject of significant investigation the mechanism and origins of selectivity in this reaction are still not completely understood.
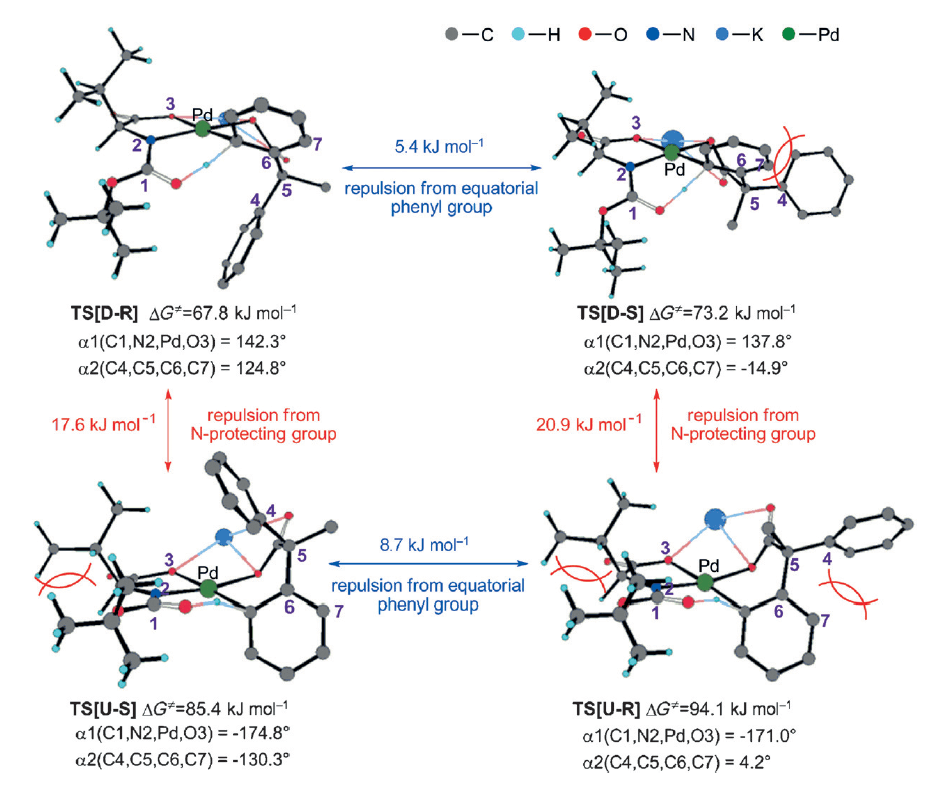
This collaborative report from the Yu and Wu groups employs a combined approach, using electrospray ionization mass spetrcoscopy (ESI-MS) and DFT calculations to gain insight. ESI-MS allowed the separation of two peaks of equal mass to identify two key transition states, which upon further investigation led them to propose that the reaction proceeds through a deprotonation from the internal nitrogen protecting group rather than an outersphere base.
Building upon these studies a new relay-based explanation for the enantioinduction is proposed, whereby the interaction between the side chain of the amino acid and the nitrogen protecting group is critical in establishing the chiral environment to organize the confirmation of the substrate.
This study suggests that careful consideration of this identified interaction could be a key factor in the design of new ligand scaffolds for future palladium catalysts.