Stereodivergent Intramolecular C(sp3)–H Functionalization of Azavinyl Carbenes: Synthesis of Saturated Heterocycles and Fused N-Heterotricycles
Vincent N. G. Lindsay , Hélène M.-F. Viart , and Richmond Sarpong
J. Am. Chem. Soc.,
2015, 137 (26), pp 8368–8371; 10.1021/jacs.5b04295
06/2015
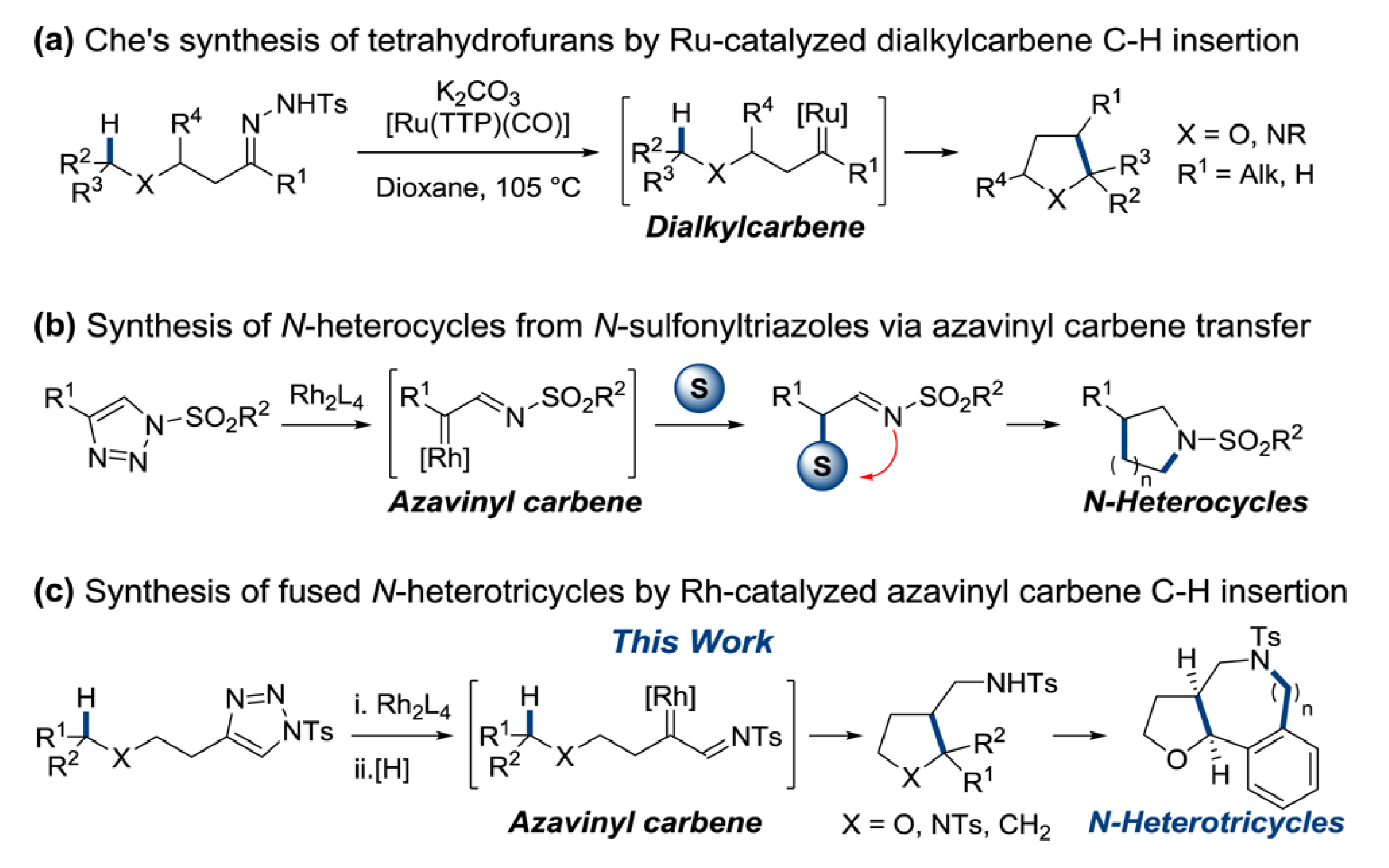
Intramolecular C–H functionalization has been established as a facile and reliable entry into heterocyclic systems. This report from the Sarpong group extend this technology, describing the synthesis of poly-substituted saturated heterocyclic systems that incorporate both aromatic and aliphatic side chains.
Using N-sulfonyltriazoles as Rh(II) azavinyl carbene equivalents a diverse array of cis-2,3-substituted tetrahydrofurans were accessible. The stereoinduction of this reaction was found to be highly dependent on the catalyst ligand environment; the greater the steric congestion, the more pronounced the preference for the cis isomer. Use of the triazole as a carbene precursor introduces a versatile functionality to the C–H functionalization product, one that the Sarpong group use to good effect to rapidly access tricyclic scaffolds reminiscent of many natural product skeletons.