Decarbonylative organoboron cross-coupling of esters by nickel catalysis
Kei Muto, Junichiro Yamaguchi, Djamaladdin G. Musaev and Kenichiro Itami
Nature Communications,
2015, 6, Article number: 7508 doi:10.1038/ncomms8508
06/2015
The Suzuki-Miyaura cross-coupling, the metal-catalyzed reaction of a boron-based nucleophile and halide-based electrophile has become a powerful and reliable tool in the construction of organic molecules, from therapeutic targets to agrochemicals and organic materials. This collaborative report from the Itami and Musaev groups describes a new approach and understanding to this chemistry, replacing the halide-based electrophile with an ester that proceeds through a decarbonylative mechanism.
This strategy has significant implications for this chemistry; using an inexpensive and sustainable nickel catalyst the substrate scope is significantly expanded and the amount of harmful halide-byproducts are eliminated.
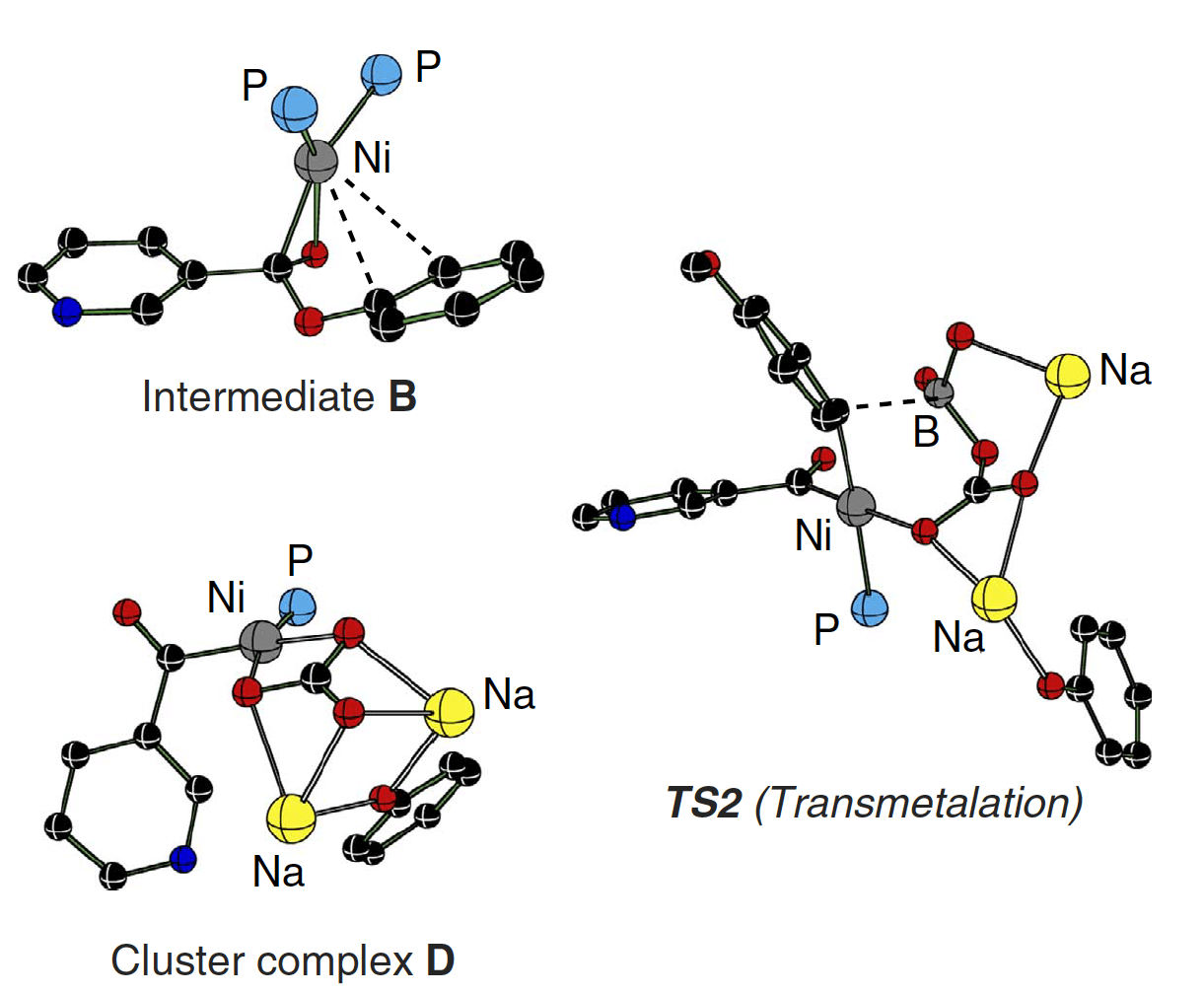
Through a combination of experimental and theoretical calculations details about the mechanism were investigated; shedding light on how the reaction can proceed both without base but is accelerated in the presence of base. It is proposed that the reaction proceeds through a transmetalation key step rather than a deprotonation and that the decarbonylation is in fact a rate-determining step in the presence of base. With no base present the transmetalation, at a slightly higher energy barrier, is found to be the rate-determining step.