Mechanistic Details of Pd(II)-Catalyzed C–H Iodination with Molecular I2: Oxidative Addition vs Electrophilic Cleavage
Brandon E. Haines, Huiying Xu, Pritha Verma, Xiao-Chen Wang, Jin-Quan Yu, and Djamaladdin G. Musaev
J. Am. Chem. Soc.,
2015, 137 (28), pp 9022–9031; 10.1021/jacs.5b03410
07/2015
Aryl halides remain one of the most versatile building block in organic chemistry, being efficient and capable coupling partners for the construction of everything from therapeutic targets to polymers. Not typically found in Nature they require synthesis and the most commonly employed method is directed lithiation.
For these reasons halogenation of C–H bonds has received significant recent interest. However this approach has remained impractical, in large part due to the impractical reagents such as IOAc, or the strong oxidants required.
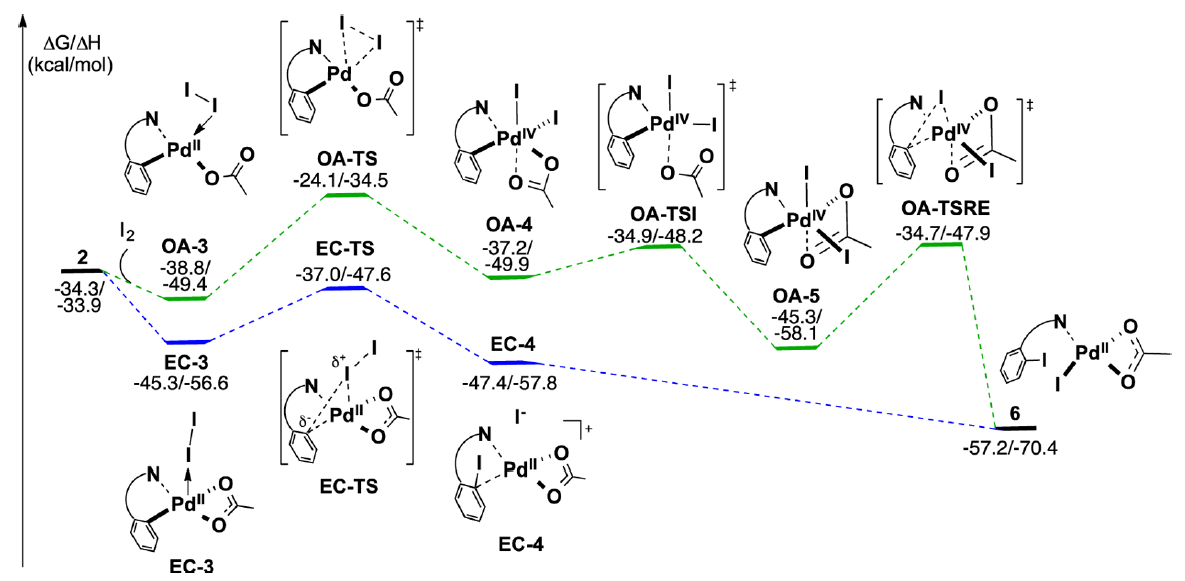
The Yu group recently disclosed a significant advance in this field, a Pd(II)-catalyzed ortho-directed C–H iodination protocol that employs molecular iodine as the sole oxidant. This collaboration between the Yu and Musaev groups uses DFT calculations to explore three critical aspects of this system a) the impact of the Pd-directing group interaction, b) the nature of the oxidant and c) the nature and hybridization of the C–H bond.
These studies describe how both the mono- and dimeric palladium complexes are competent in this transformation. It was found that the nature and strength of the interaction between the directing group and the palladium complex plays a critical role in determining the reaction pathway.