Palladium(II)-Catalyzed Allylic C–H Oxidation of Hindered Substrates Featuring Tunable Selectivity Over Extent of Oxidation
Xiangyou Xing, Nicholas R. O'Connor and Brian M. Stoltz
Angew. Chem. Int. Ed.,
2015, 54, 38, 11186-11190; 10.1002/anie.201504007
07/2015
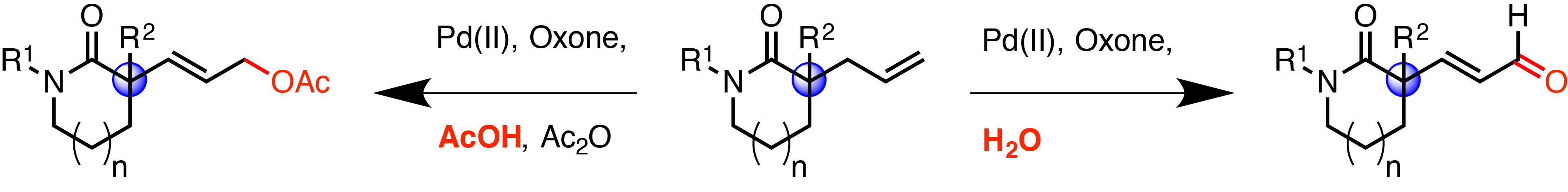
The status of the allylic C–H bond has been established as a privileged motif in the C–H functionalization literature, in large part due to the synthetic utility of allyl groups as versatile functional handles for further transformations. The Stoltz group have developed an extremely effective method for the construction of alpha-allyl lactams in excellent yield and enantioselectivity and it was thus a natural progression for the investigation of these lactams as suitable substrates for allylic C–H oxidation. However, after surveying the reported conditions it became apparent that that sterically congested nature of these molecules challenged the current C–H oxidation technology, with either no reaction or decomposition predominating.
This work focuses on the use of a directing group, the inbuilt amide functionality, to enable reactivity in this sterically hindered system. This is a tactic largely unused in allylic C–H oxygenation chemistry.
Optimization of the conditions, using oxone as the terminal oxidant has furnished and general, effective and robust technology in which the degree of oxidation can be controlled through simple modification of the reaction conditions. Essentially, either the presence or absence of water leads to a switch between a two-electron oxidationof the allylic group into the allylic acetate and a four electron oxidation of the allyl group into the enal.