Practical Pd(II)-Catalyzed C–H Alkylation with Epoxides: One-Step Syntheses of 3,4-Dihydroisocoumarins
Guolin Cheng, Tuan-Jie Li, and Jin-Quan Yu
J. Am. Chem. Soc.,
2015, 137 (34), pp 10950–10953; 10.1021/jacs.5b07507
08/2015
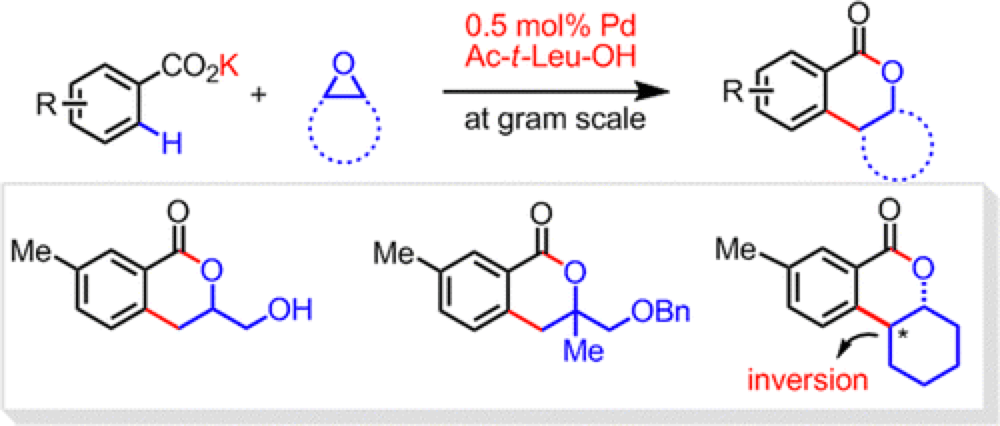
Alkylation of arenes is an essential disconnection strategy for the construction of a wide array of organic molecules, from therapeutic agents to molecular materials. Transition-metal-catalyzed alkylation has emerged as an attractive alternative to more established methods such as the Friedal-Crafts alkylation, offering significantly milder and more sustainable reaction requirements. The alkylation of arenes with alkyl halides has been extensively investigated with conditions employing Pd, Ni, Ru, Co and Fe-catalysts having been reported. Despite this body of work hurdles still remain before this reaction can fulfill its potential as a key synthetic application; the scope of substrates is limited to simple alkyl halides and the requirement of strong directing groups reduces the turnover of the catalyst systems. This report from the Yu group describes their approach to overcome these challenges.
One of the key reasons for limited substrate scope is the facile nature of the beta-hydride elimination in the alkyl halide coupling partners. A recent report from the Kanai group details the use of epoxides as coupling partners, using a pyridine as the directing functionality. Building upon this chemistry the Yu group investigated whether epoxides could be used in conjunction with the weakly-coordinating carboxylic acid directing functionality, essentially installing the same group as a more complex alkyl halide. This has previously proven difficult as alkyl halides easily add into the carboxyl group and this halts the reaction.
With soptimization of the counter-ion on the carboxyl group the Yu group have developed a highly efficient and general methodology for the introduction of a broad variety of epoxides in a stereospecific fashion, significantly extending this technology.