Function and Structure of MalA/MalA′, Iterative Halogenases for Late-Stage C–H Functionalization of Indole Alkaloids
Amy E. Fraley, Marc Garcia-Borràs, Ashootosh Tripathi, Dheeraj Khare, Eduardo V. Mercado-Marin, Hong Tran, Qingyun Dan, Gabrielle P. Webb, Katharine R. Watts, Phillip Crews, Richmond Sarpong, Robert M. Williams, Janet L. Smith, K. N. Houk, and David H. Sherman
J. Am. Chem. Soc.,
2017, 139, (34), 12060; DOI:10.1021/jacs.7b06773
07/2017
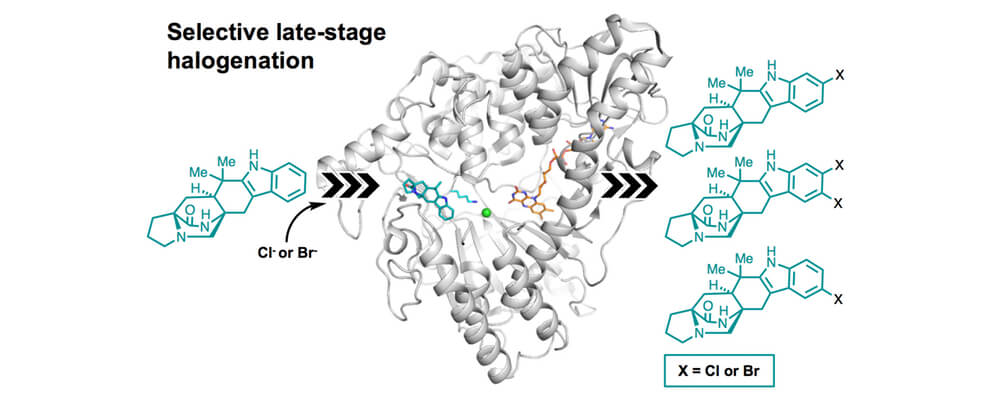
Malbrancheamide is a complex halogenated indole alkaloid with a dichlorinated indole ring that is imperative to its biological activity. Synthetic halogenation methods for complex molecules like malbrancheamide have posed a challenge due to the abundance of chemically equivalent sites. With a large number of natural products undergoing late-stage functionalization by tailoring enzymes, there is a unique opportunity to use biocatalysis to perform difficult chemical transformations.
In this work, a flavin-dependent halogenase called MalA was characterized and used to generate a variety of halogenated malbrancheamide analogues. Structural analysis and computational studies of MalA were utilized to elucidate the enzymatic mechanism of site-selective chlorination. A serine residue positioned nearest to the inherently less favorable C8-H was shown to facilitate chlorination at this site.
Based on this mechanism, engineering of the active site region led to site selective mutants of MalA. With a new understanding of the mechanism of MalA-mediated selective halogenation, future efforts will focus on rational engineering of MalA to modulate its regioselectivity, substrate scope, and iterative function.